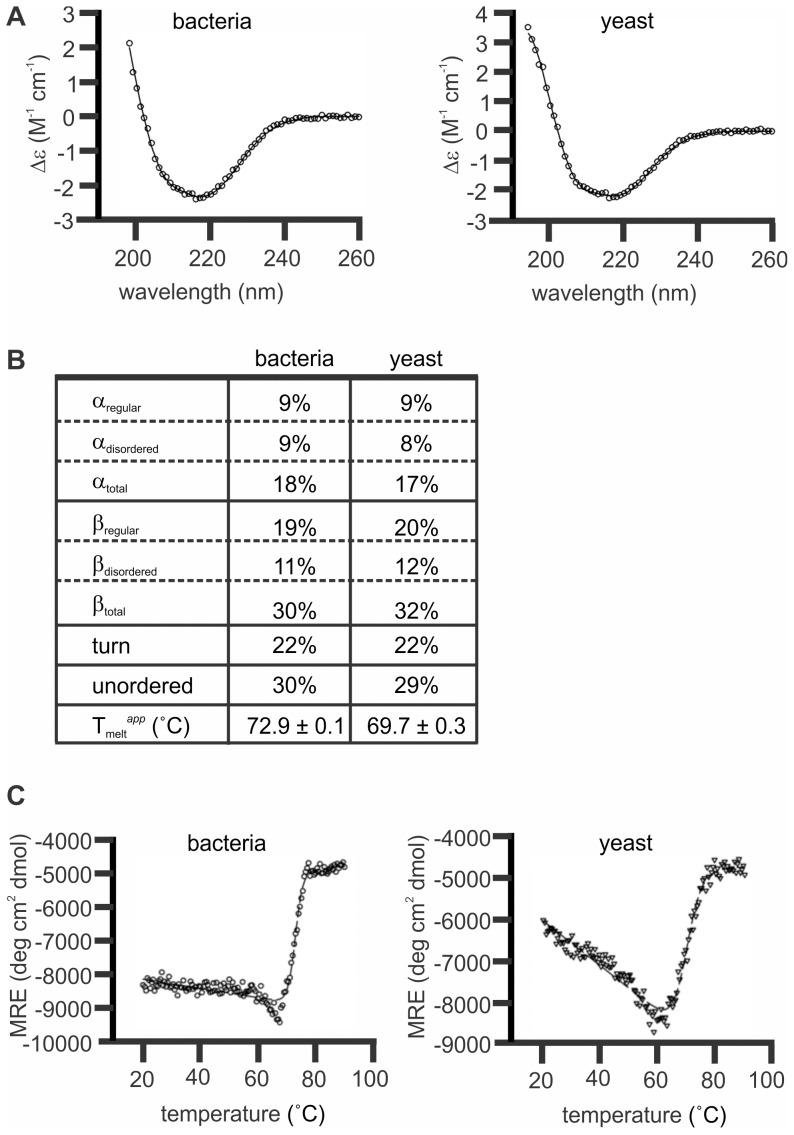
Figure 3. MaviP35 proteins purified from bacteria and yeast have similar secondary structures.
FLAG-tagged MaviP35 proteins were purified from yeast and bacteria and then subjected to circular dichroism (CD) analyses. (A) Wavelength scans were performed at 20°C. The final spectra is the average result of three scans (open circles). The CONTINLL algorithm calculated the nonlinear least squares best fit (solid line) against the SP29 protein database with r.m.s.d. values≤0.073. (B) Table of secondary structure proportions and apparent melting temperature for MaviP35 purified from bacterial and yeast. (C) Ellipticity at 216 nm was measured between 20 and 90°C (open circles). The nonlinear regression analysis (dashed lines) fitted the curves to a one step transition between folded and unfolded confirmations.