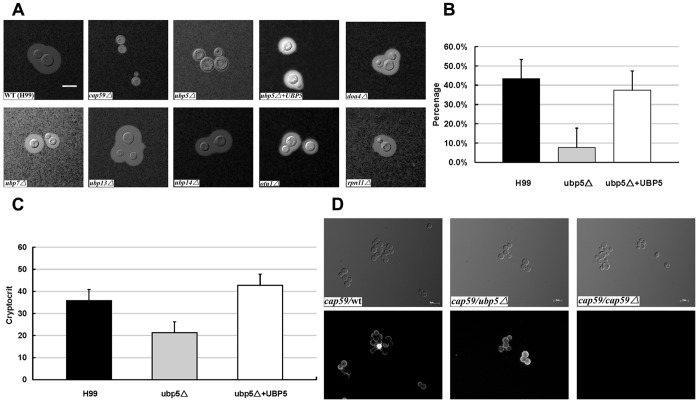
Figure 3. Disruption of UBP5 significantly down-regulates the capsule production.
A. ubp5Δ mutants have a capsule defect. The WT strain H99 and all the DUB mutants were cultured on DME medium for capsule production at 37°C for 3 days. Capsule was assessed by staining with India ink and visualizing at 100 magnification (scale bar = 10 µm). B. Relative capsule volume detection. Total (cell and capsule) and cell-only diameter were measured via Photoshop Software for 50 cells for each strain. Then calculated the relative ratio of capsule with the formula ([Total Volume-Packed Volume]/Total Volume). After statistical analysis, the capsule production of ubp5Δ strain (7.7±0.48%) significantly decreased compared with either WT (43.4±6.41%) or reconstituted strain (37.4±8.01%) (P<0.001). C. Cryptocrit for Capsule Assay. After overnight growth, each culture was inoculated into tissue culture flasks with 20 mL DMEM medium in capsule-inducing condition for three days. Cells were killed with 10% formalin (V/V) for 5 minutes, and then counted. The samples were standardized to 3.2×108 cells/mL. The difference between ubp5Δ (21.28±3.00) and complemented (42.8±0.84) or WT (35.8±1.48) strains was P<0.001. D. Capsule transfer assay. Capsule material secreted from wild-type or ubp5Δ cells into conditioned medium (CM) can be attached to acapsular acceptor cells (cap59Δ). cap59Δ cell cannot shed capsule polysaccharide, thus its CM was used as a negative control. Cells were labeled with monoclonal antibody mAb18B7 and Alexa Fluror anti-mouse IgG (secondary antibody) was used for immunofluorescence.