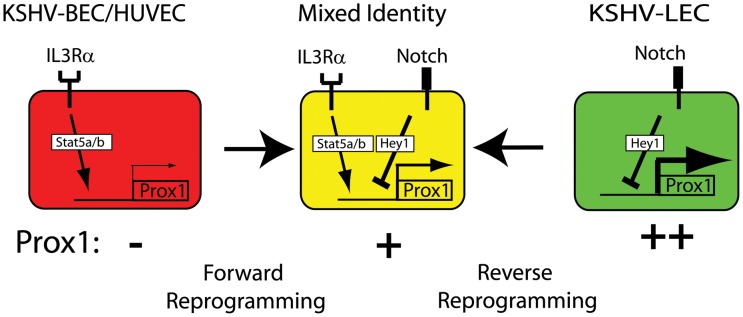
Figure 7. Working hypotheses for the KSHV-mediated bidirectional host cell fate reprogramming and for the opposing regulation of PROX1 by IL3Rα and NOTCH.
PROX1 expression level of host cells is the key determinant for the differential PROX1 regulation by KSHV. In PROX1-absent BECs and HUVECs, the PROX1-inducing IL3Rα pathway overwhelms the PROX1-repressive NOTCH pathway, resulting in PROX1 upregulation and acquisition of LEC-phenotype (Forward Reprograming). In PROX1-abundant LECs, the PROX1-repressive NOTCH pathway is more productive than the PROX1-inducing IL3Rα pathway, resulting in PROX1 downregulation and regaining BEC-phenotype (Reverse Reprograming). Importantly, however, these reprogramming processes appear to be incomplete differentiation events and thus KSHV-infected endothelial cells exhibit both (mixed) BEC and LEC-phenotypes.