Table 1.
Antibacterial activities against M. tuberculosis and S. aureus, and MenA enzyme inhibitory activities of the selected molecules.
| Compounds | Structure
|
MIC (g/mL)a |
MenA Inhibition
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| X | R1 | R2 | S. aureus | M. tuberculosis (MABA)b | M. tuberculosis (LORA)c | S. aureus (at 100 μM) | M. tuberculosis(at 100 μM) | IC50 (μM) | |
| 2 |
Class A X, R1, R2 = O, CH3, H |
>60 | 12.5 | 5.2 | − | + | 15.0 | ||
| (S)-2 | X, R1, R2 = O, CH3, H | >60 | 12.5 | 5.59 | − | + | 7.5 | ||
| (R)-2 | X, R1, R2 = O, CH3, H | >60 | 6.2 | 4.91 | − | + | 17.0 | ||
| (S)-11 | X, R1, R2 = O, C3H7, H | >60 | 12.5 | 4.91 | − | + | 16.0 | ||
| (R)-11 | X, R1, R2 = O, C3H7, H | >60 | 6.2 | 5.59 | − | + | 9.0 | ||
| (S)-12 | X, R1, R2 = O, C3H7, CONH2 | >60 | 12.5 | 2.94 | − | + | 9.0 | ||
| (R)-12 | X, R1, R2 = O, C3H7, CONH2 | >60 | 2.31 | 0.85 | − | + | 1.5 | ||
| (R)-13 | X, R1, R2 = NOMe, C3H7, CONH2 | >60 | 2.31 | 0.85 | − | + | 1.2 | ||
| 3 |
Class B X, R2 = O, H |
>60 | 12.5 | 1.88 | − | + | 15.0 | ||
| (S)-3 | X, R2 = O, H | >60 | 12.5 | 1.88 | − | + | 25.0 | ||
| (S)-3 | X, R2 = O, H | >60 | 12.5 | 1.46 | − | + | 20.0 | ||
| (S)-14 | X, R2 = O, CONH2 | >60 | 12.5 | 4.93 | − | + | 20.0 | ||
| (R)-14 | X, R2 = O, CONH2 | >60 | 12.5 | 4.93 | − | + | 15.0 | ||
| (R)-15 | X, R2 = NOMe, OH | >60 | 6.25 | 1.43 | − | + | 15.0 | ||
| 4 |
Class C |
>60 | 12.5 | 3.00 | − | + | 15.0 | ||
| (S)-4 | >60 | 12.5 | 3.00 | − | + | 17.0 | |||
| (R)-4 | >60 | 12.5 | 3.00 | − | + | 14.0 | |||
| rac-5 |
Class D X, R1, R2 = O, CH3, H |
>60 | 6.25 | 2.82 | − | + | 15.0 | ||
| rac-16 | X, R1, R2 = O, CH3, CONH2 | >60 | 3.25 | 2.83 | − | + | 7.5 | ||
| (S)-5 | X, R1, R2 = O, CH3, H | >60 | 1.50 | 1.43 | − | + | 4.5 | ||
| (R)-5 | X, R1, R2 = O, CH3, H | >60 | 6.25 | 2.85 | − | + | 7.5 | ||
| (S)-16 | X, R1, R2 = O, C3H7, H | >60 | 1.50 | 1.43 | − | + | 3.5 | ||
| (R)-16 | X, R1, R2 = O, C3H7, H | >60 | 6.25 | 5.20 | − | + | 9.5 | ||
| (S)-17 | X, R1, R2 = O, C3H7, CONH2 | >60 | 1.50 | 1.45 | − | + | 1.5 | ||
| (S)-18 | X, R1, R2 = NOMe, C3H7, CONH2 | >60 | 1.50 | 1.40 | − | + | 1.5 | ||
| rac-6 |
Class E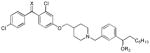 X, R2 = O, H |
>60 | 12.5 | 1.88 | − | + | 20.0 | ||
| (S)-6 | X, R2 = O, H | >60 | 12.5 | 1.88 | − | + | 15.0 | ||
| (R)-6 | X, R2 = O, H | >60 | 12.5 | 1.46 | − | + | 15.0 | ||
| (S)-19 | X, R2 = O, CONH2 | >60 | 12.5 | 2.93 | − | + | 20.0 | ||
| (R)-19 | X, R2 = O, CONH2 | >60 | 12.5 | 4.93 | − | + | 20.0 | ||
| (S)-20 | X, R2 = NOMe, OH | >60 | 12.5 | 5.54 | − | + | 20.0 | ||
| (R)-20 | X, R2 = NOMe, OH | >60 | 12.5 | 5.54 | − | + | 15.0 | ||
| RFPd | - | 0.2 | 1.47 | ||||||
| INHe | - | 0.1 | >128 | ||||||
| EMBf | - | 0.78 | >128 | ||||||
The agar plate dilution method was used.;
MABA: microplate alamar blue assay.;
LORA: Low-oxygen recovery assay.;
RFP: rifampicin.;
INH: isoniazid.;
EMB: ethanbutol.
