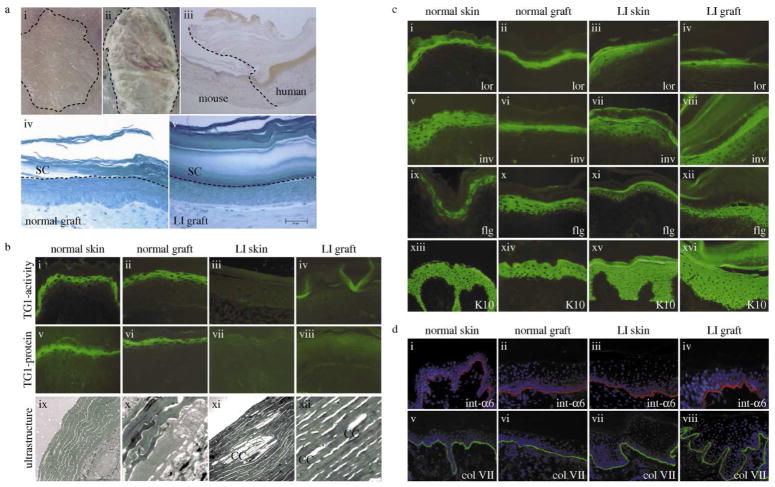
Figure 1. Characterization of the skin-humanized mouse model for transglutaminase 1-deficient lamellar ichthyosis.
(A) Keratinocytes were seeded onto a fibroblast-populated fibrin-based matrix. Skin equivalents were grafted orthotopically onto the back of athymic nude mice. (i) normal human regenerated skin and (ii) regenerated LI skin (patient 1) 12 weeks after grafting; (iii) Peroxidase staining of involucrin (human specific) confirms the human skin phenotype; (iv) semithin sections, methylene blue staining, morphology of normal and (v) LI grafts showing a very thick and packed stratum corneum. Bar: D=E = 50μm. (B) TG1-activity (i–iv), TG1-protein (v–viii) and ultrastructure (ix–xii). Normal skin/grafts show the typical pericellular distribution of TG1-activity/protein in the stratum granulosum. Ultrastructurally no cholesterol clefts are visible. In contrast, LI skin and LI grafts lacked TG1-activity/protein but displayed cholesterol clefts as typical ultrastructural markers. (C) Characterisation of human and regenerated skin by immunostaining. TG1-substrates like loricrin (i–iv), involucrin (v–viii), and filaggrin (ix–xii) in LI samples show a more diffuse and slightly shifted staining pattern when compared to the normal samples. Keratin 10 (xiii–xvi) was expressed in suprabasal layers in all four samples. (D) The distributions of integrin-α6 (i–iv) and collagen VII (v–viii) in LI skin/grafts in comparison to normal skin/grafts are visualized by immunostaining. These components of the dermoepidermal junction show a comparable staining in all samples indicating a correct formation of the junction zone. Slides were counterstained with DAPI. CC, cholesterol clefts; LI, lamellar ichthyosis; TG1, transglutaminase 1.