Figure 4.
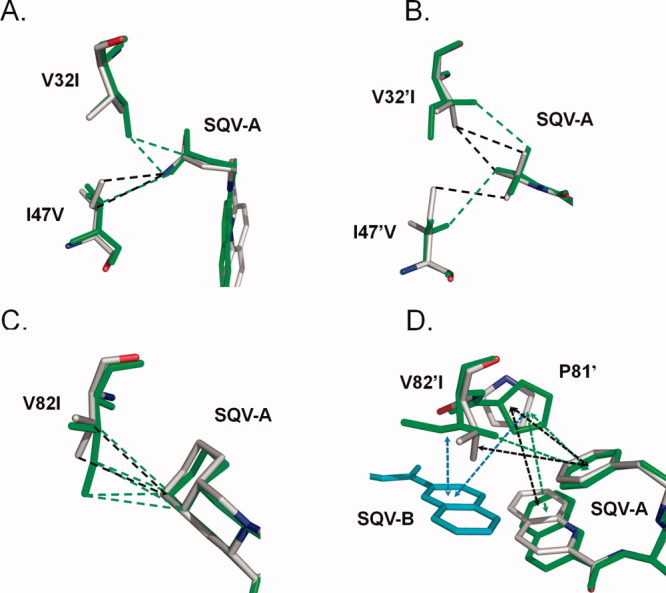
Comparison of PR1 and PR1M interactions with SQV. The PR1 structure is colored by atom type, while that of the triple mutant is shown in green bonds. Only the major conformation is shown for SQV and protein residues with alternate conformation in the PR1-SQV structure. SQV-A is the molecule bound in the regular active site cavity. Hydrophobic interactions (distances of 3.3–4.2 Å between non-hydrogen atoms) are indicated as dashed lines. Interactions with aromatic groups (CH…π) are indicated by dashed arrows. (A) Residues 32 and 47 of subunit A; (B) Residues 32′ and 47′ of subunit B; (C) Residue 82; (D) Residue 82′. SQV-B (cyan bonds) indicates the extra SQV molecule. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
