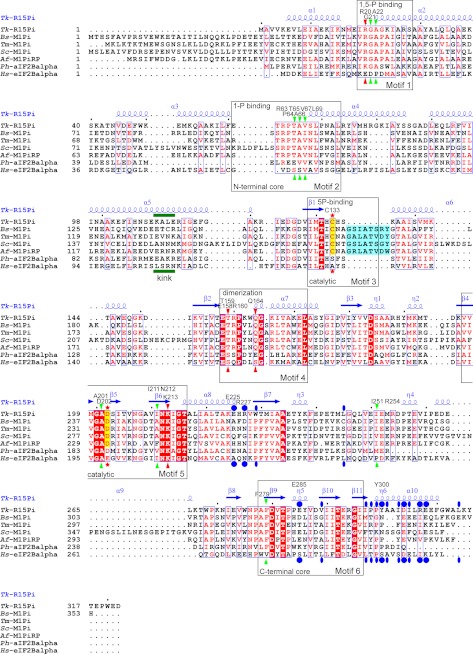
FIGURE 4.
Amino acid sequence alignment of the structure-determined PF01008 family proteins. Sequences were aligned by ClustalW (40), and this figure was produced by ESPript (41) with manual modifications. The names of species and UniProt accession numbers are as follows: Tk-R15Pi, R15Pi from T. kodakarensis KOD1, Q5JFM9; Bs-M1Pi, M1Pi from B. subtilis, O31662; Tm-M1Pi, M1Pi from Thermotoga maritima, Q9X013; Sc-M1Pi, M1Pi from Saccharomyces cerevisiae, Q06489; Af-M1PiRP, M1Pi-related protein from A. fulgidus, O29877; Ph-aIF2Balpha, archaeal IF-2B α-subunit from Pyrococcus horikoshii OT3, O58185; Hs-eIF2Balpha, eukaryotic IF-2B α-subunit from Homo sapiens, Q14232. Identical and similar amino acid residues are highlighted by white characters in red closed boxes and red characters in blue open boxes, respectively. The residues highlighted in blue are the insertion regions related to the substrate binding of M1Pi. The catalytic residues of R15Pi and M1Pi (e.g. Cys133 and Asp202 of Tk-R15Pi) are indicated by red stars and are also highlighted in yellow. Red and green arrowheads represent important residues constructing the active site surrounding Cys133 and Asp202, respectively. These residues are considered to contribute in the deprotonation or protonation of Cys133 or Asp202, respectively. Blue closed circles, residues related to the hexamerization by hydrophilic interactions, including salt bridges. Blue closed ellipses, residues related to the hexamerization by hydrophobic interaction. The bend region of the α5 helix is indicated by the word kink with a green bar. The six sequence motifs described in this work are boxed, and their major roles are indicated. The secondary structures of Tk-R15Pi are indicated above the sequence alignment. Residue names and numbers of Tk-R15Pi described in this work are also shown above the sequence alignment.