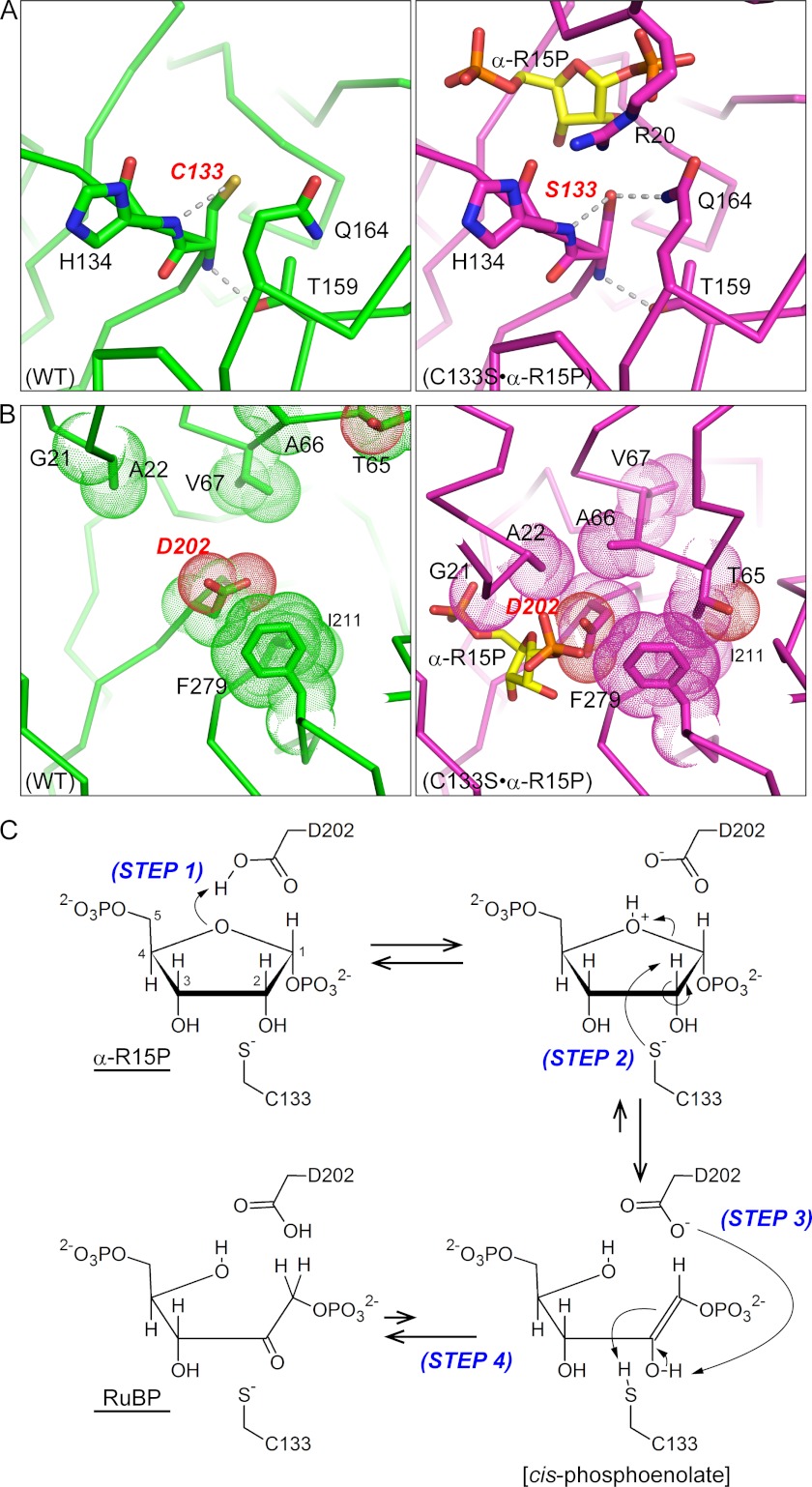
FIGURE 7.
Proposed isomerase reaction model of Tk-R15P. The protein environments around Cys133 (A) and Asp202 (B) are shown. Significant conformational changes from the open (WT, green, left panels) to the closed conformation (C133S·α-R15P, magenta, right panels) allow Tk-R15Pi to enter a reaction-ready state. C, proposed reaction mechanism via a cis-phosphoenolate intermediate. Step 1, proton transfer from the protonated carboxylate of Asp202 to the O4 atom of α-R15P; Step 2, proton abstraction from the C2 atom of α-R15P by the deprotonated thiolate of Cys133 and the cleavage of the C1–O4 bond; Step 3, proton abstraction from the O2 atom of the cis-phosphoenolate intermediate by deprotonated carboxylate of Asp202 and proton transfer from the thiol of Cys133 to the C1 atom of the intermediate; Step 4, production of RuBP by keto-enol tautomerization.