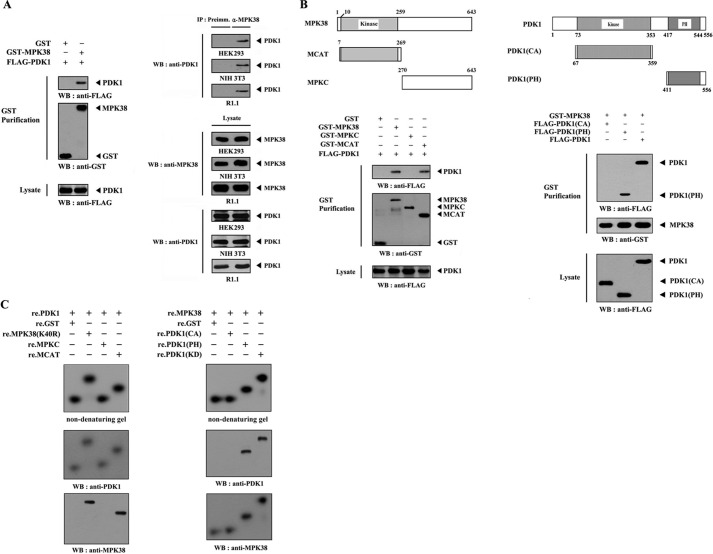
FIGURE 1.
MPK38 interacts with PDK1. A, in vivo association of MPK38 with PDK1. GST alone or GST-MPK38 was cotransfected into HEK293 cells along with FLAG-PDK1. GST fusion proteins were purified on glutathione-Sepharose beads (GST Purification), and complex formation was analyzed by immunoblotting with an anti-FLAG antibody (left panel). Equal amounts of cell lysate from HEK293, NIH 3T3, and R1.1 cells were immunoprecipitated with either rabbit preimmune serum (Preimm.) or anti-MPK38 antibody (α-MPK38) followed by immunoblot analysis with an anti-PDK1 antibody to determine endogenous binding (right top panels). B, mapping of the MPK38 and PDK1 binding domains. The schematic structures of wild-type and deletion constructs of Mpk38 (left panel) and PDK1 (right panel) are shown. HEK293 cells transfected with the indicated expression vectors were lysed, precipitated using glutathione-Sepharose beads, and then immunoblotted with an anti-FLAG antibody to determine the level of MPK38-PDK1 binding. C, in vitro binding of MPK38 and PDK1. For native PAGE of the MPK38-PDK1 complex, autophosphorylated His-tagged PDK1 or MPK38 (each 2–3 μg), prepared in the presence of the respective kinase buffers (24, 29), were incubated with unlabeled recombinant GST-tagged kinase-dead MPK38 (or PDK1) and its deletion constructs (for MPK38, MPKC, and MCAT; for PDK1, CA, and PH) (each 5 μg), together with the nonspecific control GST at room temperature for 1 h. The same blot was stripped and re-probed with anti-PDK1 and anti-MPK38 antibodies to confirm the presence of PDK1 and MPK38 on the radioactive band shifts (middle and bottom panels). The purity of recombinant PDK1 and MPK38 proteins used for this experiment was analyzed by Coomassie Blue staining (see supplemental Fig. 4A). IP, immunoprecipitation; re., recombinant; WB, Western blot; MPK38(K40R), kinase-dead MPK38; Preimm., preimmune serum.