FIGURE 2.
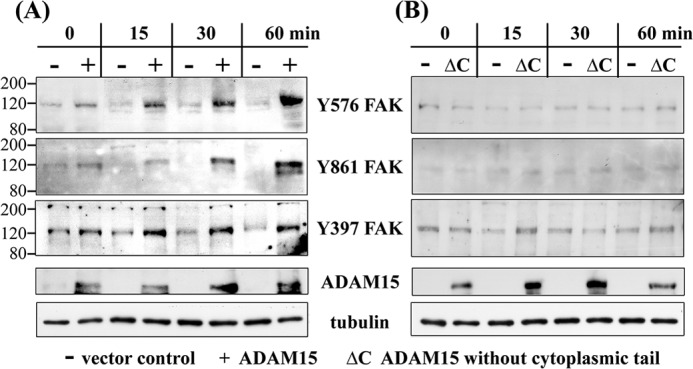
Enhanced phosphorylation of FAK in ADAM15-transfected chondrocytes after apoptosis induction by camptothecin. A, immunoblots of cell lysates of vector-transfected (−) and ADAM15-transfected cells (+) treated with 2 μm camptothecin for up to 60 min, showing a stronger phosphorylation at Tyr-576, Tyr-861, and Tyr-397 of FAK in the ADAM15-expressing cells in comparison with vector-transfected cells. B, T/C28a4 cells transfected with ADAM15 lacking the cytoplasmic tail (ΔC), however, did not display an activation of FAK. Blots were controlled for ADAM15 and/or ADAM15ΔC expression, and loading was monitored using anti tubulin antibodies. The immunoblots are representative of at least five repeated experiments.
