FIGURE 3.
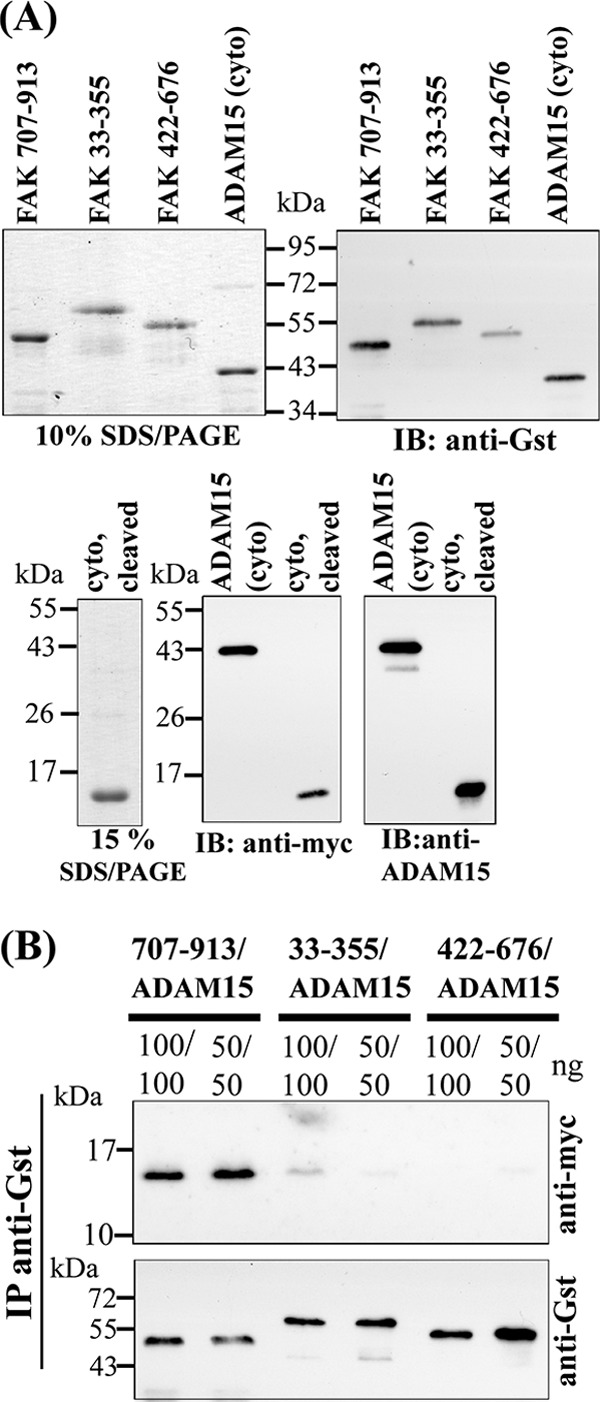
Generation of recombinant ADAM15 and FAK proteins for protein binding assays. A, upper left panel: Coomassie stained SDS-PAGE of the purified Gst-tagged FAK fragments and the Gst- and Myc-tagged cytoplasmic domain of ADAM15 (cyto) and upper right panel: the detection of these proteins with anti-Gst antibodies by Western blotting. A, lower panels: SDS-PAGE of the cytoplasmic domain of ADAM15 with the Gst tag cleaved off by PreScission Protease, followed by immunodetection using anti-Myc and anti-ADAM15 antibodies. B, pulldown assays: cell lysates spiked with Myc-tagged ADAM15 were co-incubated with either of the three different FAK proteins. Upon immunoprecipitation (IP) using anti-Gst-Sepharose and subsequent immunoblotting (IB), bound ADAM15 was detected by anti-Myc antibodies. Blots were stripped, and the Gst-tagged FAK proteins were verified by anti-Gst antibodies, thereby demonstrating the interaction of ADAM15 with the C-terminal FAK-region (amino acids 707–913).
