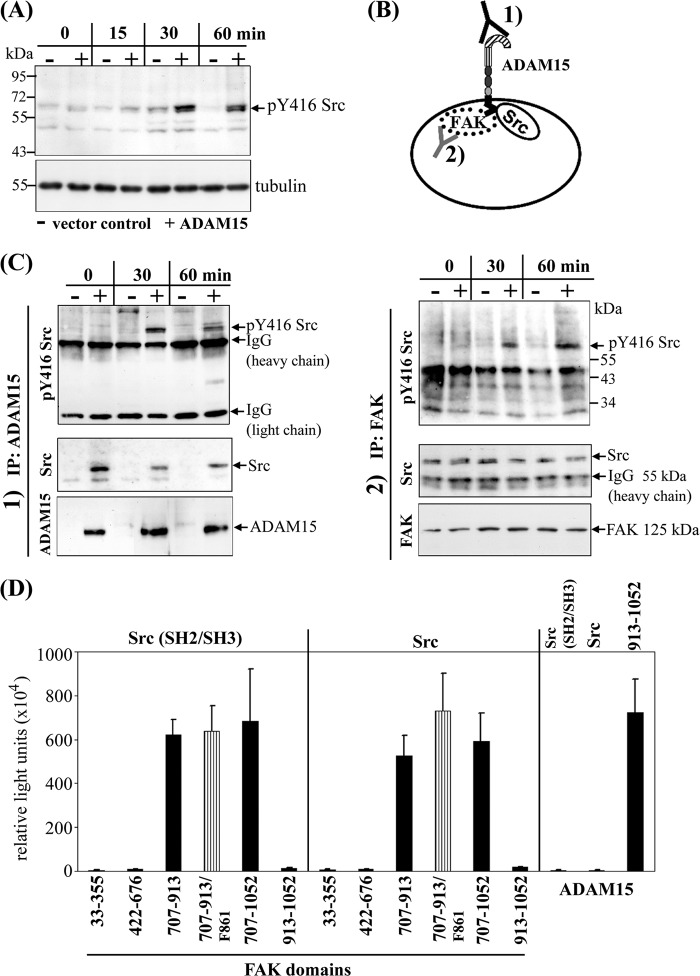
FIGURE 6.
Stronger phosphorylation of Src kinase in ADAM15-transfected cells is dependent on direct binding to FAK. A, upon stimulation with camptothecin for 60 min, an enhanced phosphorylation of Tyr-416 Src was detected in whole cell lysates of ADAM15-transfected cells (+) as compared with those derived from respective vector control cells (−) by immunoblotting. B, to analyze whether the stronger phosphorylation of Src results from an interaction with ADAM15 or with FAK, co-immunoprecipitations (IP) were performed by using as the precipitating antibody either 1) anti-ADAM15 or 2) anti FAK. C, lysates of ADAM15 (+) and vector control cells (−) upon camptothecin exposure were immunoprecipitated with either anti-ADAM15 1) or anti-FAK antibodies 2) and immunoblotted using anti Tyr-416 Src antibodies. A stronger phosphorylation of Tyr-416 Src was observed in the ADAM15-transfected cells compared with the vector control cells, independent of the antibody used for the precipitation. Blots were stripped, and precipitated Src was visualized using anti-Src antibodies. Src was pulled down in ADAM15 precipitated lysates only (vector-transfected cells, negative). In all anti-FAK antibody-precipitated lysates, equal amounts of Src protein were demonstrated. Blots were restripped and controlled for precipitated ADAM15 1) or FAK 2) using respective antibodies. D, mammalian two-hybrid was used to analyze the binding partner of Src. The N-terminal SH2/SH3 domain as well as full-length Src were cloned into the prey vector and distinct FAK domains into bait vector. Bait and prey were co-transfected with the firefly luciferase reporter and a Renilla luciferase control plasmid into HEK cells. A strong binding of the SH2/SH3 domain as well as full-length Src to the C-terminal region of FAK (707–913) was measured, whereas the FERM, the catalytic, and the FAT domain did not bind to Src. Mutation of Tyr-861 into Phe-861 of FAK did not affect Src interaction. However, no direct binding of ADAM15 to SH2/SH3 Src or full-length Src could be detected by contrast to the already proven interaction with the C-terminal FAK domain (707–1052, Fig. 4) that served as a positive control in this experiment.