FIGURE 7.
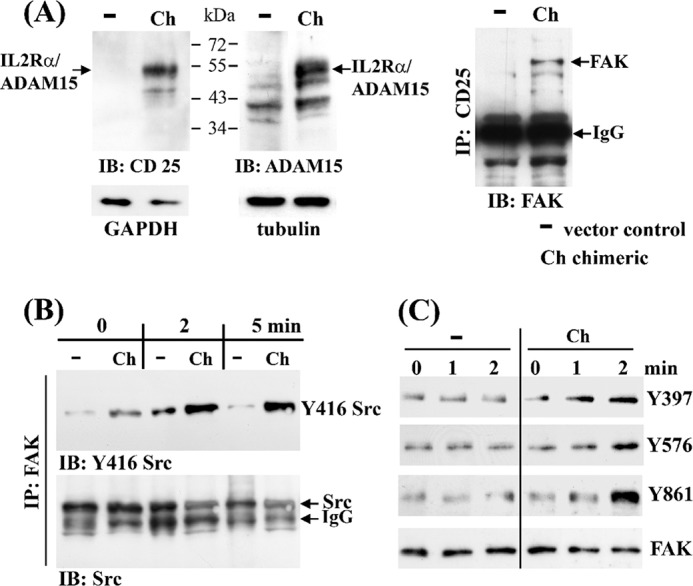
Signaling of ADAM15 expressed as a IL-2 receptor-α/cytoADAM15 chimera. The T/C28a4 chondrocyte cell line was stably transfected with a chimeric construct composed of the extracellular IL-2 receptor-α (CD25) fused to the cytoplasmic tail of ADAM15, and the chimeric protein was detected by immunoblotting using either anti-CD25 or anti-ADAM15 antibodies at ∼60 kDa (A, left panels). A, right panel, shows co-immunoprecipitation of FAK only in cell lysates of cells transfected with the chimeric IL2Rα/cytoADAM15 construct (Ch) as compared with vector transfected cells (−) using anti-CD25 antibodies. B, immunoprecipitation (IP) using anti-FAK antibodies show co-precipitated Src in both vector and chimera-transfected cells, but an enhanced phosphorylation of Tyr-416 Src is noted exclusively in the IL2Rα/cytoADAM15 cells following stimulation with IL-2. C, immunoblots (IB) of lysates derived from IL-2 stimulated cells exhibit a stronger phosphorylation of FAK at Tyr-397, Tyr-576, and Tyr-861 in IL2Rα/cytoADAM15 cells. Equal loading was controlled by reprobing with anti-FAK antibodies.
