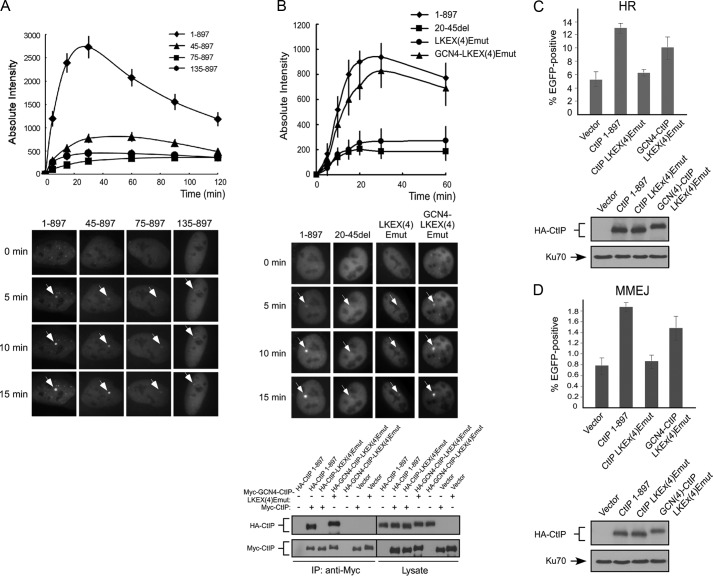
FIGURE 6.
CtIP dimerization is important for its recruitment to DSBs. A, U2OS cells were transiently co-transfected with EGFP-CtIP 1–897 or the indicated N-terminal deletion mutants and mRFP-PCNA, and live cell imaging of S phase cells, marked by PCNA S phase-associated replication foci, was performed on cells following laser-induced microirradiation using a pulsed nitrogen laser. Top panel, absolute intensity of EGFP-CtIP fluorescence signal at damage sites was calculated. The error bars indicate the S.D. Bottom panel, accumulation of EGFP-CtIP WT or indicated N-terminal deletion mutants at laser-induced damage sites are shown by white arrows at the indicated time points. B, U2OS cells were transiently co-transfected with EGFP-CtIP 1–897 or indicated mutants and mRFP-PCNA, and live cell imaging of S phase cells, marked by PCNA S phase-associated replication foci, was performed on cells following laser-induced microirradiation using a picosecond short pulsed green laser. Top panel, absolute intensity of EGFP-CtIP fluorescence signal at damage sites was calculated using Image J software, with an average of 10 independent measurements shown. The error bars indicate the S.D. Middle panel, accumulation of EGFP-CtIP 1–897 or indicated mutants at laser-induced damage sites are shown by white arrows at the indicated time points. Bottom panel, 293T cells were co-transfected with Myc-CtIP 1–897 (wild type) or Myc-GCN4-CtIP-LKEX4Emut and HA-CtIP 1–897 or indicated mutants. Anti-Myc immunoprecipitation (IP) was performed, followed by anti-HA Western blotting. C and D, U2OS EGFP-HR or U2OS EGFP-MMEJ cells stably expressing HA-CtIP 1–897 or indicated mutants, with endogenous CtIP silenced by shRNA and siRNA, were transfected with I-SceI-IRES-dsRedNLS, and 72 h after transfection, the cells were trypsinized, and FACS analysis was performed. The data shown represent the means of three independent experiments; the error bars indicate the S.D. Western blot shows expression of HA-CtIP 1–897 and mutants, with Ku70 used as a loading control.