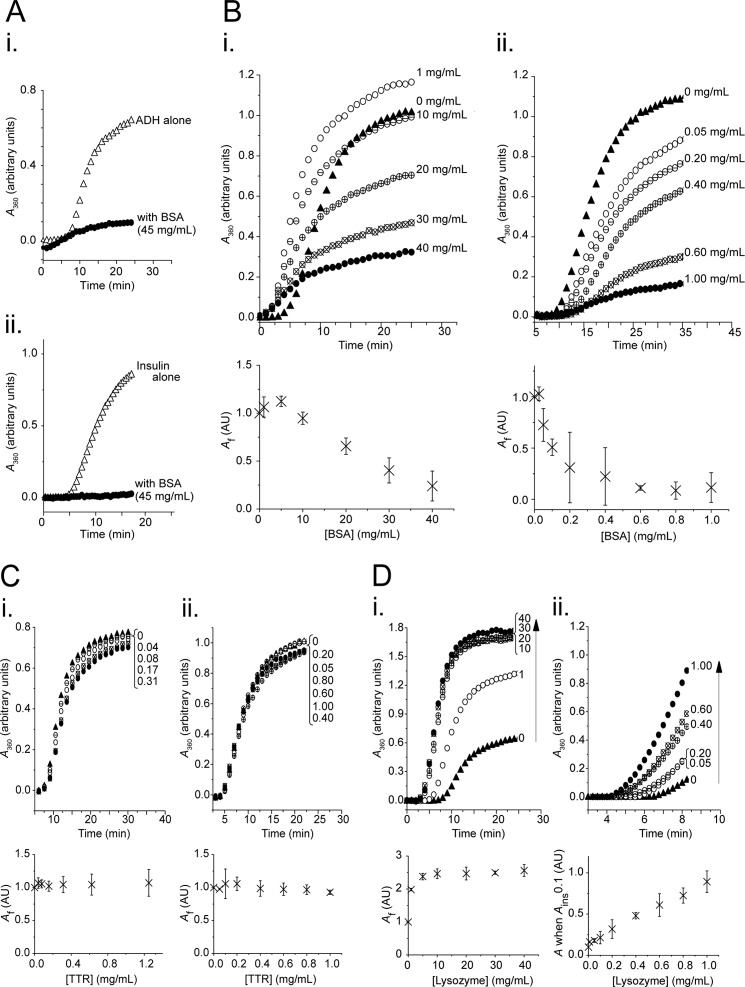
FIGURE 1.
BSA displays chaperone-like activity at physiological concentrations, and its protection of ADH and insulin is titratable. Aggregation assays contained ADH (350 μg/ml) at 55 °C (panels i) or insulin (350 μg/ml) at 37 °C with 15 mm DTT (panels ii) in the absence (triangles) or presence (circles) of BSA (A and B), transthyretin (C), or lysozyme (D) at concentrations as indicated (mg/ml). Aggregation was monitored as A360, with a representative data set from two or more independent experiments shown in the upper panel, and the mean final aggregation (Af) from kinetic analysis of the pooled data (± 95% confidence interval) shown in the lower panel. A, chaperone-like activity of physiological concentrations of BSA (45 mg/ml): protection of ADH (panel i) and insulin (panel ii). B, chaperone-like activity of BSA lends titratable protection to ADH (panel i) and insulin (panel ii) against aggregation. C, transthyretin (control), when present at physiological levels does not protect either ADH (panel i) or insulin (panel ii) against aggregation. D, lysozyme (control) at concentrations matched to BSA does not protect either ADH (panel i) or insulin (panel ii) against aggregation.