FIGURE 5.
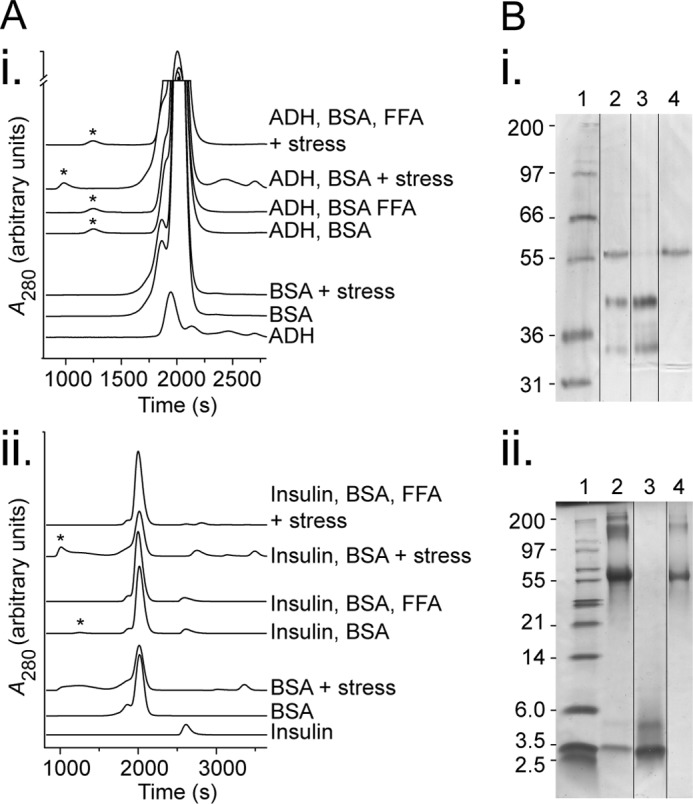
BSA forms stable high molecular weight species with stressed target proteins. A, size exclusion analysis of BSA and target proteins. Solutions of individual proteins or mixtures of BSA, FFAs (683 μm of physiological mixture), and target protein (ADH at 800 μg/ml (panel i) or insulin at 500 μg/ml (panel ii)) were left in their native state or stressed (45 °C for 60 min, or 37 °C with 15 mm DTT for 10 min, respectively). The product was then filtered to remove precipitated proteins and analyzed by size exclusion chromatography, and asterisks indicate the excluded volume from the column (different gel filtration matrices gave rise to slightly shifted elution profiles for the first, third, and fourth traces (top down in each)). B, SDS-PAGE of excluded volume peaks from stressed target protein with BSA conditions in A. The samples were run in Tris-Tricine gels and stained with silver stain (panel i) or reduced with DTT prior to loading and stained with Coomassie Blue (panel ii) with lanes as follows: standard (lane 1, band sizes indicated in kDa), excluded volume from condition stressed target protein with BSA (lane 2), target protein (lane 3), and BSA (lane 4). Lane order was rearranged as demarcated for ease of comparison. The data are representative of three independent experiments.
