Table 1.
Protection of the uridine ureido nitrogen with 1 and its relativities against representative reagents.
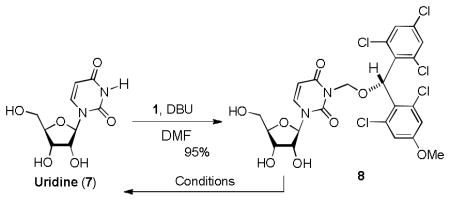 | |||
|---|---|---|---|
| Conditionsa | 8 (t1/2) | Conditionsa | 8 (t1/2) |
| 20% TFA, CH2Cl2 | >6h | DBU, Toluene | >24h |
| 10% TMSOTf, CH2Cl2 | >2h | Al(Hg), 1,4-Dioxane | >12h |
| 10% HCl, MeCN | >6h | Raney Ni 1,4-Dioxane | >12h |
| 10% HCl, 1,4-dioxane | >6h | nBu3SnH, AIBN Toluene, reflux | >6h |
| 30% HF, MeCN | >12h | NBS THF |
>6h |
| 80% AcOH | >12h | ||
| 30% TsOH, 1,4-dioxane | >12h | hv, MeCN | >6h |
| La(OTf)3, THF | >12h | H2/Pd-C, 1 atom MeOH | >12hb |
| 10% BF3-OEt2, CH2Cl2 | >12h | H2/Pd-C, 50 psi MeOH | >12hb |
| 10% TiCl4, CH2C12 | >4h | H2/Pd-C, 100 psi MeOH | >12hb |
| DDQ, CH2Cl2-water | >12h | H2/Pd black MeOH | >12hb |
Reaction was carried out at room temperature.,
the uracil double bond of 8 was reduced.
