Table 2.
Scope of the one-pot cyclopentane synthesis with alcohols 2.
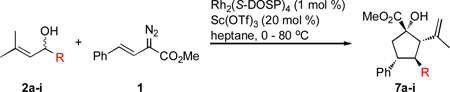 | |||||
|---|---|---|---|---|---|
| entry | comp. | R | yielda (%) |
drb | eec (%) |
| 1 | a | Me | 95 | > 20 : 1 | 82 |
| 2 | b | i-Pr | 67 | > 20 : 1 | 80 |
| 3 | c | i-Bu | 73 | > 20 : 1 | 80 |
| 4 | d | n-Hex | 80 | > 20 : 1 | 78 |
| 5 | e | 86 | > 20 : 1 | 76 | |
| 6 | f | 45d | > 20 : 1 | 90 (96)e | |
| 7 | g | 65f | > 20 : 1 | 78 | |
| 8 | h | Bn | 42 | > 20 : 1 | 87 |
| 9 | i | 59 | > 20 : 1 | 84 | |
Isolated yield.
Determined from 1H NMR of crude reaction mixture.
Determined by chiral HPLC.
Yield of the deprotected ketone.
Number in parentheses indicates ee of recrystallized product.
Reaction conducted in refluxing heptane in the absence of Sc(OTf)3.
