Figure 1.
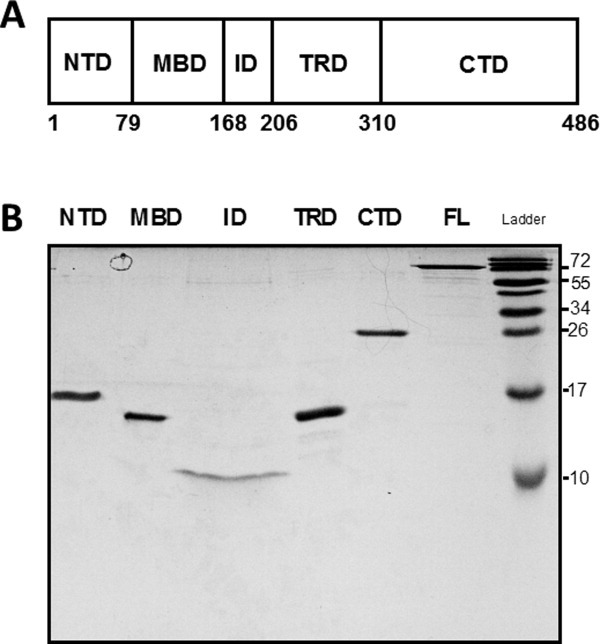
(a) Schematic illustration of the domain organization of MeCP2. NTD, N-terminal domain; MBD, methyl DNA binding domain; ID, intervening domain, TRD, transcriptional repression domain, CTD, C-terminal domain. Residue numbers of the domain boundaries are shown at the bottom. (b) SDS-PAGE of MeCP2 and its isolated domains. Full-length (FL) MeCP2 and its domains were electrophoresed on a 20% SDS-polyacrylamide gel for 120 min at 100 V. Because of different sample concentrations a larger volume of the ID was loaded relative the other domains to achieve approximately comparable band intensities, leading to horizontal band spreading. Note that full-length MeCP2 and all isolated domains exhibit anomalously slow migration during SDS-PAGE, consistent with their intrinsically disordered nature.2
