Figure 2.
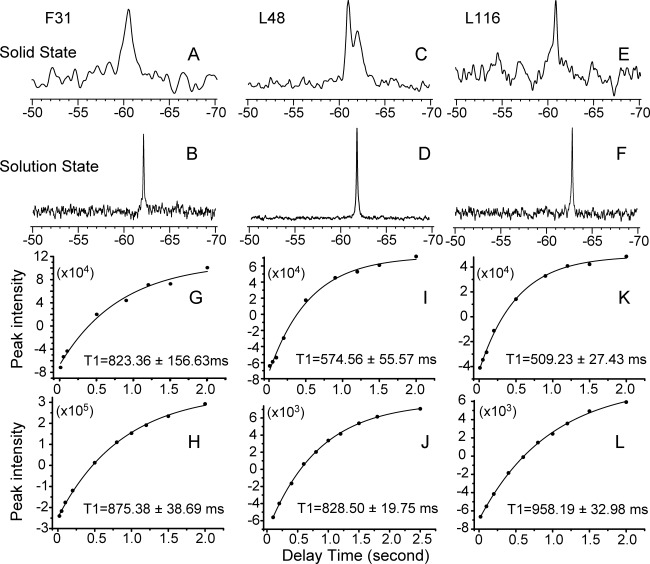
In situ magic angle spinning solid state NMR 19F chemical shifts (A, C, E) and longitudinal relaxation T1 values (G, I, K) were obtained for the DAGK protein in its native E. coli membrane without protein purification. Solution NMR 19F chemical shifts (B, D, F) and longitudinal relaxation T1 values (H, J, L) were obtained for samples of purified DAGK in DPC micelles. Three 19F-tfmF site-specifically labeled DAGK proteins were presented: F31-tfmF (A, B, G, H); L48-tfmF (C, D, I, J) and L116-tfmF (E, F, K. L).
