Abstract
Human empathy is not merely a resonance with others’ physical condition, but is modulated by social factors. Using functional magnetic resonance imaging, the present study demonstrated an increased brain empathic response to others in pain when they received no rather than a large reward, with increments of the ACC, aMCC, insula and postcentral gyrus in the pain matrix and temporoparietal junction. Thus, pain target’s financial situation modulated brain empathic responses in the pain matrix based on an understanding of the situation pain target faces.
Keywords: empathy, pain, monetary reward, aMCC, insula
In the psychological literature, empathy usually refers to the capacity to understand and share the emotional and affective states of another person in relation to oneself (Decety and Jackson, 2004; Lieberman, 2007; Hein and Singer, 2008). According to the perception–action model of empathy (Preston and de Waal, 2002), observing or imagining others in a particular emotional state activates a representation of that state in the observer. The hypothesis of shared representations between self and other has been shown by a growing number of neuroimaging and neurophysiology studies on empathy for pain, which have demonstrated that the perception of others’ pain activates similar regions of the pain matrix observed in the first-hand experience of pain (Derbyshire, 2000; Jackson et al., 2006b), including both areas for encoding the motivational–affective dimension of pain, such as bilateral anterior insula (AI), anterior cingulate cortex (ACC) and the anterior mid-cingulate cortex (aMCC) (e.g. Morrison et al., 2004, 2007a, b; Singer et al., 2004; Botvinick et al., 2005; Jackson et al., 2005, 2006a, b; Gu and Han, 2007; Lamm et al., 2007a, b; Moriguchi et al., 2007; Saarela et al., 2007; Akitsuki and Decety, 2009; Danziger et al., 2009), and areas for encoding the sensory dimension of pain, such as the somatosensory cortex (e.g. Avenanti et al., 2005; Bufalari et al., 2007; Lamm et al., 2007b; Moriguchi et al., 2007; Valeriani et al., 2008; Akitsuki and Decety, 2009). Altogether, there is converging evidence to suggest that perception of others’ pain triggers a resonance mechanism between other and self (Cheng et al., 2007).
However, as social animals, the full-blown empathy capacity of human is more complex than a mere resonance with the target’s painful state (Decety et al., 2008). Recent brain imaging studies demonstrated that human empathy for pain was modulated by social factors, such as the affective link between individuals (Singer et al., 2006), the intentionality of the perceived agency who induced the pain (Decety et al., 2008; Akitsuki and Decety, 2009), the racial membership of the target compared to the observer (Xu et al., 2009), prior attitudes toward the targets based on their stigmatized status (Decety et al., 2010), and the facial expression of the pain targets (Han et al., 2009). The present study aimed at elucidating the effect of another social factor, i.e. the financial situation of the target person in the painful situation, on observers’ empathic responses. Direct empirical evidence for the role of such social factor in empathic perception and response to others allows key insights into the nature of the empathy system.
In considering how empathic responses to others’ pain might be modulated by social factors, receipt of money by the sufferer may be important. Recent studies have demonstrated that the mere idea of wealth induced by money primes can bring about a feeling of self-sufficiency which makes participants less likely to offer or request help (Vohs et al., 2006). Money may promote people’s feelings of strength and efficacy to achieve physical safety and psychological security (Zhou and Gao, 2008). As empathy enables a better understanding of the mental states of others (Lieberman, 2007; Rameson and Lieberman, 2009), it is predicted that the self-sufficiency of people in a better financial situation makes others believe that they have the ability to overcome difficulties and pain, which leads to less empathy for them and a reduced neural empathic response accordingly when they are enduring pain. In contrast, the less self-sufficiency of poor people makes others give more understanding and empathic responses to them. Thus, leaving aside the increased neural empathic response in pain-related regions (e.g. AI, ACC, aMCC), observing poor people in pain will also cause increased engagement of the temporoparietal junction (TPJ), which has been held to play a key role in understanding others’ intentions, beliefs and actions from others’ perspectives (Saxe and Kanwisher, 2003; German et al., 2004; Vollm et al., 2006; Williams et al., 2006; Decety and Lamm, 2007; Overwalle, 2009).
Besides, another insight into how the financial situation of the target person in the painful situation modulates observers’ empathic responses comes from researches about schadenfreude. Instead of understanding the self-sufficiency of rich people, observers could also feel jealous of rich people, and accordingly, ignore or even enjoy the rich people’s pain. Thus, a rich people’s pain may cause pleasure, a phenomenon termed as ‘schaudenfreude’ (Smith et al., 2009; Sundie et al., 2009). If this is true, an increased activation in schaudenfreude-related areas (e.g. ventral striatum, Cikara et al., 2011) could be observed when viewing rich people in pain.
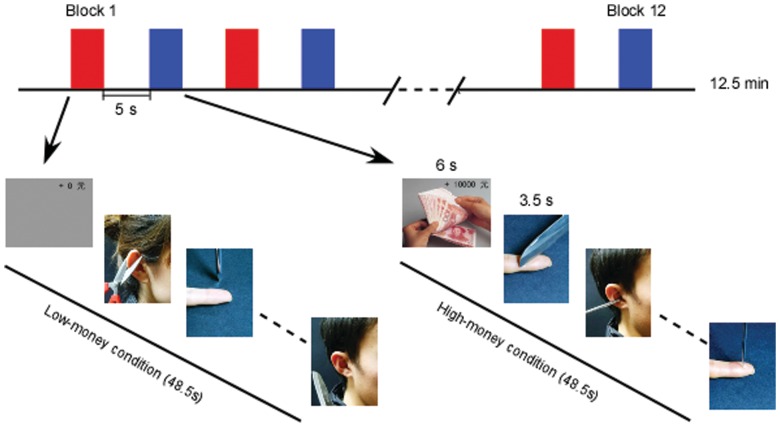
To test this hypothesis, we conducted an fMRI study to examine whether the hemodynamic responses in the pain-related neural networks, TPJ and ventral striatum were modulated by the financial situation of pain targets. During the experiment, participants were scanned while viewing a set of pictures showing individuals in painful or non-painful scenes. The financial situation of the pained individuals was indicated by a cue before each block (Figure 1). The low-money condition denoted individuals in the following pictures received no money before their pain experience, whereas the high-money condition denoted individuals in the following pictures received 10 000 RMB (∼1471 US dollars) before their pain experience. It is hypothesized that, if an increased activation in pain-related regions and TPJ were observed when individuals experiencing pain received no rather than large payment, then the reduced empathic responses could be attributed to perspective taking explanation. However, the findings that viewing rich people, but not poor people in pain engaged a reduced activation in pain-related regions and an increased activation in ventral striatum would give support for schaudenfreude explanation.
Fig. 1.
A hybrid-design paradigm was used. Each money condition contained six blocks (red for low-money condition and blue for high-money condition), with a 5 s rest between each block. Before each block, there was a 6 s cue to inform participants of the financial situation of pain targets (how much money they got) in this block. In each block, four painful pictures and four non-painful pictures randomly presented with null trials, each lasting 3.5 s. The interstimulus intervals (ISI) were jittered from 0.5 to 1.5 s. A black fixation cross was presented during the intervals and null trials. Different money condition blocks were alternate between each other and the presentation order of the blocks sequence being counterbalanced across participants.
METHOD
Participants
A total of 16 right-handed participants (11 female, aged from 20 to 29 years, M = 23.5, s.d. = 3.43) participated in this experiment. All the participants were recruited from the university community and paid 100 RMB for their participation. None of them had a history of neurological or psychiatric disorders. All participants had normal or corrected-to-normal vision and gave informed consent before scanning.
Materials
Ninety-six pictures showing left index finger and right ear in painful and non-painful situations (48 each) were used as stimuli. Painful situations depicted four kinds of nociceptive stimulations (cutting the finger or ear by a knife or a pair of scissors and pricking the finger or ear by a needle or an awl). A non-painful situation was paired with each of eight painful situations, in which the nociceptive tool did not touch the finger or ear, but was laid aside from the body part (Figure 1). All pictures were 300 × 400 pixels in size.
For each of 16 kinds of situations, half of the six pictures were used in the low-money condition; and the others in the high-money condition. Thus, the 96 pictures were divided to 4 categories (24 in each category), including: painful situations in the low-money condition (PL), non-painful situations in the low-money condition (NL), painful situations in the high-money condition (PH) and non-painful situations in the high-money condition (NH).
Procedure
A hybrid design paradigm was used in the study, with six blocks for each money condition. Each block contained four painful pictures and four non-painful pictures which were displayed on a gray background, randomly interspersed with null events. During null events, the fixation cross remained on screen. Each trial was presented for 3.5 s with jittered inter-stimulus intervals (ISI) from 0.5 to 1.5 s, during which a black fixation cross was presented against the gray background. Different money condition blocks were alternate between each other, with the presentation order of the blocks being counterbalanced across participants (ABABABABABAB for half of participants and BABABABABABA for the others). Each block lasted for 48.5 s with a 5 s rest between blocks. Before each block, a 6 s cue trial was displayed to inform the participants which money condition the following block belonged to. The participants were asked to watch the pictures attentively and try to experience the feelings of the owner of the body part in the picture. They were instructed that there was no relationship between the money they obtained and the pain they received. After a structural scan, pictures were presented on a screen that could be seen by means of mirrors placed on the head coil.
After being scanned, the participants repeated the same viewing procedure with the same stimuli in the same sequence as in the scanner and were asked to rate the level of pain and unpleasantness that they thought the individual in the pictures was experiencing by a 10-point Likert-type scale from no pain to extreme pain and no effect to extreme unpleasantness, where 0 indicated no pain or no effect and 10 indicated extreme pain or extreme unpleasantness.
fMRI image acquisition and analysis
Scanning was performed on a 3T Siemens Trio system (East China Normal University, Shanghai) with a standard head coil to obtain functional images using a gradient echo echo-planar imaging (EPI) sequence. Thirty-five transversal slices covering the whole brain were acquired sequentially with a 0.3 mm gap (TR = 2200 ms, TE = 30 ms, FOV = 220 mm, flip angle = 90°, matrix size = 64 × 64, slice thickness = 3 mm, gap = 0.3 mm). There was one run of functional scanning which was ∼13 min (342 EPI volumes). Before the functional run, a high-resolution structural image was acquired using a T1-weighted, multiplanar reconstruction sequence (MPR) (TR = 1900 ms, TE = 3.42 ms, 192 slices, slice thickness = 1 mm, FOV = 256 mm, flip angle = 9°, matrix size = 256 × 256).
Data preprocessing was carried out with SPM5 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, London, UK) implemented in MATLAB. The first five volumes were discarded to allow for T1 equilibration effects. During preprocessing, images were first realigned to the first volume to correct for interscan head movements, and then the mean EPI image of each subject was computed and spatially normalized to the MNI single subject template. The normalizing parameters were applied to the functional images, which were re-sampled to 2 × 2 × 2 mm voxel size. The data were then smoothed with a Gaussian kernel of 8 mm full-width half-maximum to accommodate intersubject anatomical variability.
Statistical analyses were then performed using the general linear model (GLM) implemented in SPM5. An event-related design was used at the first level analysis with four types of events (PL, PH, NL and NH). Events were convolved with a canonical hemodynamic response function (HRF) and its time derivatives. All the events were modeled as 3.5 s long from the onset time of the pictures. The models additionally included all the cues and six movement parameters derived from realignment as covariates of no interest. High pass temporal filtering with a cutoff of 180 s was also applied in the models. For each subject at the first-level analysis, simple main effects for each of the four conditions were computed by applying the ‘1 0’ contrasts. The four first-level individual contrast images were then analyzed at the second group level employing a random-effects model (flexible factorial design in SPM5).
The main effect of pain was computed by contrasting PL and PH trials with NL and NH trials to identify pain-related activations. The main effect of monetary reward was calculated by comparing PH and NH trials with the PL and NL trials to identify brain regions corresponding to monetary reward. And the interaction [(PL−NL)−(PH−NH)] contrast was carried out to extract specific regions showing increased activations when individuals experiencing pain received no rather than large payment. A voxel-level threshold of P < 0.001 (uncorrected) and a spatial extent threshold of k > 50 were used. To further test our prior hypothesis that neural responses to others’ pain are modulated by their financial situation, we defined regions of interest (ROIs) in pain-related regions and TPJ based on the related contrasts of pain in Singer et al. (2004) and understanding others in Williams et al. (2006). ROIs were defined as 6-mm spherical regions centered on the peak or local maximum coordinate in the activated clusters and their parameter estimates were extracted for further statistics using the MarsBaR toolbox in SPM5. Finally, regions showing significant correlation between brain BOLD signal change in the (painful vs non-painful) contrast and corresponding behavioral rating difference were defined separately for the low money and high-money conditions with a voxel-level threshold of P < 0.005 (uncorrected) and a spatial extent threshold of k > 15.
RESULTS
Behavioral data
Table 1 shows the means (s.d.’s) for the pain intensity and unpleasantness ratings. A 2(pain: painful vs non-painful) × 2(financial situation: low money vs high money) repeated-measure ANOVA on the ratings of pain intensity and ratings of pain unpleasantness revealed significant main effects of pain [intensity: F(1,15) = 472.29, P < 0.01; unpleasantness: F(1,15) = 213.60, P < 0.01] and significant main effects of financial situation [intensity: F(1,15) = 6.24, P < 0.05; unpleasantness: F(1,15) = 39.64, P < 0.01], indicating higher ratings for painful situations (vs non-painful situations) and for the low-money condition (vs high-money condition). For ratings of pain unpleasantness, the interaction between money and pain was also significant [F(1,15) = 9.20, P < 0.01]. The difference of pain unpleasantness ratings between painful situations and non-painful situations in the low-money condition (M = 5.83, s.d. = 1.68) was significantly higher than in the high-money condition (M = 4.93, s.d. = 1.49), indicating that the participants’ empathy for others’ pain were modulated by the amount of money others have.
Table 1.
Means (±s.d.) for pain intensity ratings and unpleasantness ratings
| Pain intensity |
Pain unpleasantness |
|||
|---|---|---|---|---|
| Low money | High money | Low money | High money | |
| Painful | 7.49 ± 1.04 | 7.02 ± 1.29 | 7.38 ± 1.65 | 5.45 ± 1.70 |
| Non-painful | 0.73 ± 0.99 | 0.40 ± 0.57 | 1.55 ± 0.95 | 0.52 ± 0.56 |
fMRI results
Main effect of pain
The significant BOLD signal increase when viewing painful situations vs non-painful situations [(PL + PH) − (NL + NH)] was observed in a similar neural network in previous studies on the empathy of others’ pain, including aMCC, L ACC (L: left; R: right), bilateral SMA (supplementary motor area), insula extended to inferior frontal gyrus, somatosensory cortex and thalamus (Table 2), indicating the normal empathic brain response of participants to others’ pain.
Table 2.
Regions showing the main effect of pain
| Region of activation | Lat. | Coordinates |
T-score | k | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Postcentral gyrus | L | −60 | −20 | 34 | 9.47 | 2183 |
| Inferior frontal gyrus | L | −32 | 28 | −6 | 8.45 | 3826 |
| Insula | L | −34 | 8 | 0 | 6.66 | |
| SMA | L/R | 0 | 16 | 52 | 8.09 | 3302 |
| Middle cingulate cortex | R | 8 | 26 | 34 | 5.96 | |
| Middle cingulate cortex | L | −6 | 22 | 38 | 5.39 | |
| ACC | L | −2 | 16 | 28 | 4.98 | |
| Inferior frontal gyrus | R | 50 | 14 | 2 | 7.90 | 2578 |
| Insula | R | 44 | 8 | 0 | 6.37 | |
| SupraMarginal gyrus | R | 62 | −24 | 36 | 5.59 | 1405 |
| Postcentral gyrus | R | 60 | −20 | 30 | 5.49 | |
| Inferior occipital gyrus | L | −44 | −68 | −4 | 5.52 | 647 |
| Pallidum | R | 14 | 2 | −4 | 5.20 | 301 |
| Inferior temporal gyrus | R | 58 | −66 | −8 | 4.54 | 237 |
| Thalamus | L | −12 | −12 | 6 | 4.48 | 206 |
| Precentral gyrus | R | 38 | 0 | 46 | 3.98 | 154 |
Note. Coordinates (mm) are in MNI space. L = left hemisphere; R = right hemisphere. P < 0.001 (uncorrected), k ≥ 50.
Main effect of monetary reward
Data analyses revealed greater activation in regions including left ventral striatum and medial and lateral prefrontal cortex during high-money trials relative to low-money trials by contrasting [(PH + NH) − (PL + NL)] (Table 3). These results conformed to prior demonstrations of ventral striatum and prefrontal activations in the context of monetarily rewarding tasks (Bush et al., 2002; Knutson et al., 2003; Scott et al., 2007; Wrase et al., 2007). Contrarily, the reverse contrast revealed significant activation in TPJ, indicating more understanding and greater perspective taking to poor people.
Table 3.
Regions showing the main effect of monetary reward
| Region of activation | Lat. | Coordinates |
T-score | k | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| (PH + NH) − (PL + NL) | ||||||
| Inferior frontal gyrus | L | −52 | 20 | 8 | 6.90 | 919 |
| Ventral striatum | L | −28 | 4 | −4 | 4.50 | |
| Middle temporal gyrus | R | 68 | −12 | −18 | 6.81 | 812 |
| Superior frontal gyrus | R | 14 | 54 | 26 | 6.49 | 6755 |
| Superior frontal gyrus | L | −34 | 58 | 0 | 6.14 | |
| Middle orbital gyrus | L | −32 | 56 | −4 | 6.01 | |
| Mid orbital gyrus | R | 10 | 60 | −4 | 4.94 | |
| Angular gyrus | R | 50 | −50 | 34 | 5.86 | 1010 |
| Middle frontal gyrus | L | −34 | 22 | 36 | 5.67 | 897 |
| Inferior temporal gyrus | L | −54 | −6 | −30 | 4.90 | 1130 |
| Middle frontal gyrus | R | 38 | 26 | 42 | 4.56 | 395 |
| Inferior frontal gyrus | R | 50 | 30 | −10 | 4.22 | 226 |
| (PL + NL) − (PH + NH) | ||||||
| Fusiform Gyrusg | R | 42 | −40 | −22 | 6.41 | 997 |
| Inferior temporal gyrus | R | 56 | −52 | −20 | 5.37 | |
| Inferior temporal gyrus | L | −54 | −54 | −16 | 5.82 | 943 |
| Postcentral gyrus | L | −22 | −50 | 58 | 5.77 | 2863 |
| Superior parietal lobule | L | −32 | −50 | 58 | 5.70 | |
| Middle occipital gyrus | R | 32 | −74 | 30 | 5.36 | 741 |
| Inferior parietal lobule | R | 36 | −40 | 46 | 5.17 | 1155 |
| Insula | L | −40 | 2 | 10 | 4.56 | 51 |
| Thalamus | L | −14 | −26 | 2 | 3.85 | 55 |
| Temporoparietal junction | R | 60 | −26 | 28 | 3.63 | 94 |
Coordinates (mm) are in MNI space. L = left hemisphere; R = right hemisphere. P < 0.001(uncorrected), k ≥ 50.
Interaction between pain and monetary reward
To identify regions showing increased activations when individuals experiencing pain received no rather than large payment, [(PL − NL) − (PH − NH)] contrast was calculated. Consistent with our prediction, regions in aMCC, SMA, insula and somatosensory cortex of the pain matrix and regions in TPJ resulted from the analysis (Table 4), which was in support of the perspective taking explanation for increased empathic neural responses to poor people. It should be noted that additional activations were also observed in dorsal striatum, which implied that pain effects in dorsal striatum were evident following the low-money condition, but not following the high-money condition. These results were consistent with previous demonstration that dorsal striatum engaged during painful related to non-painful trials (Lamm et al, 2007a, b; Danziger et al., 2009). However, the reverse contrast revealed no significant activation. Inconsistent with the schaudenfreude explanation, no ventral striatum regions survived even when we reduced the threshold to P < 0.05 (uncorrected).
Table 4.
Regions showing interaction between pain and monetary reward
| Region of activation | Lat. | Coordinates |
T-score | k | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Dorsal striatum | L | −20 | 4 | 8 | 7.41 | 35 222 |
| Middle cingulate cortex | L | −6 | 16 | 38 | 7.01 | |
| Insula lobe | L | −34 | 8 | 0 | 6.61 | |
| Middle cingulate cortex | R | 8 | 12 | 42 | 6.51 | |
| Postcentral gyrus | L | −40 | −14 | 36 | 6.19 | |
| ACC | L | −8 | 24 | 28 | 6.18 | |
| SMA | R | 8 | 0 | 64 | 5.97 | |
| Temporoparietal junction | R | 48 | −32 | 26 | 4.49 | |
| Inferior temporal gyrus | L | −50 | −22 | −20 | 5.23 | 334 |
| Middle temporal gyrus | L | −54 | −52 | 4 | 4.99 | 134 |
| Fusiform gyrus | L | −22 | −42 | −18 | 4.46 | 104 |
| Precentral gyrus | L | −38 | −4 | 62 | 4.28 | 68 |
| Superior temporal gyrus | R | 54 | −16 | −4 | 4.19 | 136 |
Note. Coordinates (mm) are in MNI space. L = left hemisphere; R = right hemisphere. P < 0.001(uncorrected), k ≥ 50.
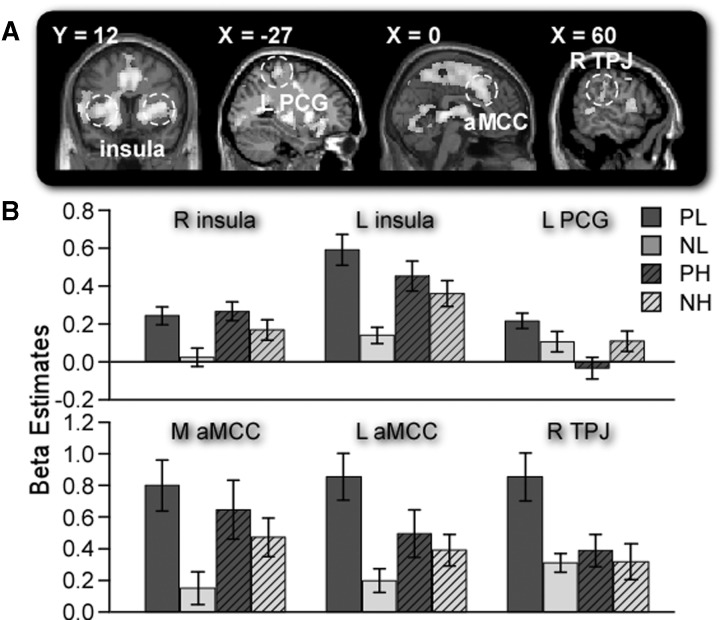
ROI analysis
ROIs in medial anterior middle cingulate cortex (M aMCC: 0 27 33), L aMCC (−3 12 42), bilateral insula (L: −36 12 0; R: 33 21 −9), left postcentral gyrus (L PCG: −27 −39 60) and R TPJ (60 −28 28) were defined according to previous studies (Singer et al., 2004; Williams et al., 2006) and beta estimates for all four trial types in each ROI were extracted (Figure 2). Several 2 pain × 2 monetary reward ANOVAs on beta estimates revealed significant main effects of pain in R insula, L insula, M aMCC, L aMCC and R TPJ (F’s > 14.95, P’s < 0.002), significant main effects of monetary reward in L PCG and R TPJ (F’s > 6.94, P’s < 0.02) and significant interactions in all ROIs (F’s > 5.87, P’s < 0.03). Combined with the above results, this finding demonstrated that both regions in pain matrix and TPJ showed increased activations when individuals experiencing pain received no rather than large payment.
Fig. 2.
(A) Regions of interest [M aMCC (0 27 33), L aMCC (−3 12 42), bilateral insula (L: −36 12 0; R: 33 21 −9), L PCG (−27 −39 60) and R TPJ (60 −28 28)] based on previous studies. L = left hemisphere; R = right hemisphere. (B) Beta estimates for all four trial types in each ROI, showing greater BOLD signal change in the low-money condition than that in the high-money condition. Error bars indicate s.e.m.
Correlation analysis
First, correlation analyses were performed to determine the regions whose BOLD signal change detected from the (PL−NL) contrast varied with corresponding average rating difference of pain intensity and unpleasantness between painful situations and non-painful situations in the Low-money condition, respectively. Interestingly, we again observed clusters located in SMA and L ACC that showed strong correlation with ratings of pain intensity (SMA: r = 0.75, L ACC: r = 0.57, P < 0.05 with Bonferroni correction for multiple comparison, complete list of clusters shown in Table 5) and clusters located in L PCG and L TPJ that showed strong correlation with ratings of pain unpleasantness (L PCG: r = 0.66, L TPJ: r = 0.62, P < 0.05 with Bonferroni correction for multiple comparison). Second, similar correlation analyses were performed in high-money condition. Table 5 displays all the activated clusters, including middle frontal gyrus that showed strong correlation with ratings of pain unpleasantness (r = 0.56, P < 0.05).
Table 5.
Regions showing correlation between differential activations during painful and non-painful trials and behavioural rating difference
| Region of activation | Lat. | Coordinates |
T-score | k | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Pain rating in low-money condition | ||||||
| Inferior temporal gyrus | L | −46 | −6 | −34 | 6.99 | 43 |
| Middle occipital gyrus | R | 38 | −92 | 6 | 5.85 | 91 |
| Inferior frontal gyrus | R | 52 | 20 | 10 | 4.93 | 187 |
| Middle frontal gyrus | R | 34 | 4 | 56 | 4.66 | 262 |
| SMA | L/R | −4 | 24 | 62 | 4.47 | 152 |
| Precentral gyrus | L | −46 | −6 | 32 | 4.40 | 48 |
| Superior temporal gyrus | R | 58 | −20 | 0 | 4.24 | 214 |
| Linual gyrus | L | −18 | −80 | −12 | 4.17 | 69 |
| Fusiform gyrus | R | 42 | −22 | −30 | 4.15 | 26 |
| ACC | L | −6 | 14 | 24 | 4.05 | 16 |
| SupraMarginal gyrus | R | 64 | −34 | 46 | 3.96 | 48 |
| Superior temporal gyrus | L | −56 | −22 | 2 | 3.94 | 26 |
| Precuneus | R | 12 | −56 | 54 | 3.93 | 15 |
| Dorsal striatum | L | −18 | 6 | 6 | 3.82 | 49 |
| Inferior frontal gyrus | L | −42 | 14 | 24 | 3.73 | 31 |
| Thalamus | R | 18 | −12 | 2 | 3.53 | 38 |
| Cuneus | R | 16 | −68 | 34 | 3.52 | 41 |
| Superior frontal gyrus | L | −16 | 64 | 20 | 3.25 | 17 |
| Unpleasantness rating in low-money condition | ||||||
| Inferior frontal gyrus | R | 40 | 38 | −2 | 5.67 | 99 |
| Superior temporal gyrus | R | 60 | −22 | 2 | 4.76 | 53 |
| SupraMarginal gyrus | R | 66 | −30 | 42 | 4.68 | 84 |
| Precuneus | R | 14 | −54 | 54 | 4.43 | 30 |
| ParaHippocampal gyrus | R | 36 | −18 | −28 | 4.12 | 49 |
| Postcentral gyrus | L | −30 | −28 | 50 | 3.99 | 39 |
| Middle cingulate cortex | R | 16 | −30 | 42 | 3.95 | 96 |
| Hippocampus | R | 26 | −4 | −24 | 3.76 | 109 |
| Amygdala | R | 28 | −2 | −22 | 3.58 | |
| TPJ | L | −64 | −48 | 28 | 3.47 | 20 |
| Inferior temporal gyrus | R | 44 | −54 | −14 | 3.42 | 29 |
| Pain rating in high-money condition | ||||||
| Superior parietal lobule | R | 36 | −60 | 64 | 6.62 | 44 |
| Middle occipital gyrus | R | 30 | −88 | 10 | 4.32 | 88 |
| Fusiform gyrus | R | 34 | −72 | −12 | 3.99 | 30 |
| Inferior occipital gyrus | L | −44 | −68 | −4 | 3.62 | 30 |
| SMA | L | −14 | 16 | 66 | 3.52 | 31 |
| Unpleasantness rating in high-money condition | ||||||
| Middle frontal gyrus | R | 40 | 10 | 48 | 3.75 | 33 |
| Inferior parietal lobule | R | 50 | −48 | 48 | 3.53 | 35 |
Note. Coordinates (mm) are in MNI space. L = left hemisphere; R = right hemisphere. P < 0.005 (uncorrected), k ≥ 15.
DISCUSSION
The results of the present study showed that perception of others in painful situations (relative to non-painful situations) was associated with significant BOLD signal increase in aMCC, L ACC, bilateral insula, SMA, somatosensory cortex and thalamus, confirming the striking overlap of neural networks between first-hand pain experience and pain empathy observed in pervious brain imaging studies, including both the affective and the sensory dimensions of the pain matrix (Cheng et al., 2007; Lamm et al. 2007b). It is worth mentioning that consistent with earlier studies (Jackson et al., 2005; Akitsuki and Decety, 2009), ACC and PCG demonstrated significant correlation between brain BOLD signal change in the (PL−NL) contrast and corresponding rating difference, but insula did not, which might be due to relatively small sample size of 16 subjects. More importantly, we found significantly decreased neural responses (ACC, aMCC, insula and PCG) for targets receiving large rather than no monetary rewards, accordingly indicating that empathy for pain was probably modulated by the targets’ financial situation. This notable finding provides further evidence that human empathy not only involves bottom-up resonance with another’s state, but also top-down information processing during which social factors affect the bottom-up empathic processing (e.g. Singer et al., 2006).
As an important social resource which enables individual to obtain benefits and satisfy needs, money can confer a broad feeling of self-confidence and efficacy to cope with various problems (Kesebir and Hong, 2008; Zhou and Gao, 2008; Zhou et al., 2009). Consistently, Zhou et al. (2009) found that participants’ ratings for pain intensity decreased after having them counting money, suggesting that the mere priming of money can relieve pain (Kreuzbauer and Chiu, 2008). In our experiment, when viewing wealthy others in pain, the perception of the good financial situation may inhibit people’s empathic neural responses accordingly, through the belief that wealthy people have enough resources and confidence to cope with physical pain by themselves. Conversely, when viewing poor others in pain, the perception of the bad financial situation may warrant more empathy relative to wealthy people as understanding their disadvantaged role in social wealth. The findings that TPJ showed increased activation when viewing poor people in pain and significant correlation with behavioral rating differences in Low-money condition gave evidence for this perspective taking explanation.
In conclusion, the present study demonstrates the increased empathic responses in aMCC, insula PCG and TPJ for people in a worse financial condition, suggesting that target’s financial situation modulated brain empathic responses in the pain matrix based on an understanding of the situation pain target faces. The results complement previous observations that empathic neural responses are modulated by various kinds of social factors (e.g. Decety et al., 2008; Xu et al., 2009), indicating top-down processing in the human empathy system.
Conflict of Interest
None declared.
Acknowledgments
This research was supported by National Natural Science Foundation of China (30870782), Key Projects of Philosophy and Social Sciences Research, Ministry of Education (06JZD0039) and Fundamental Research Funds for the Central Universities.
REFERENCES
- Akitsuki Y, Decety J. Social context and perceived agency modulate brain activity in the neural circuits underpinning empathy for pain: an event-related fMRI study. NeuroImage. 2009;47:722–34. doi: 10.1016/j.neuroimage.2009.04.091. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nature Neuroscience. 2005;8:955–60. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expression of pain engages cortical areas involved in the direct experience of pain. NeuroImage. 2005;25:312–9. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cerebral Cortex. 2007;17:2553–61. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, et al. Dorsal anterior cingulate cortex: a role in rewardbased decision making. Proceedings of the National Academy of Sciences. 2002;99:523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Lin C, Liu HL, et al. Expertise modulates the perception of pain in others. Current Biology. 2007;17:1708–13. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Cikara M, Botvinick MM, Fiske ST. Us versus them: social identity shapes neural responses to intergroup competition and harm. Psychological Science. 2011;22:306–13. doi: 10.1177/0956797610397667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61:203–12. doi: 10.1016/j.neuron.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Decety J, Echols S, Correll J. The blame game: the effect of responsibility and social stigma on empathy for pain. Journal of Cognitive Neuroscience. 2010;22:985–97. doi: 10.1162/jocn.2009.21266. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. The Neuroscientist. 2007;13:580–93. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y. Who caused the pain? A functional MRI investigation of empathy and intentionality in children. Neuropsychologia. 2008;46:2607–14. doi: 10.1016/j.neuropsychologia.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Derbyshire SWG. Exploring the pain ''neuromatrix''. Current Review of Pain. 2000;4:467–77. doi: 10.1007/s11916-000-0071-x. [DOI] [PubMed] [Google Scholar]
- German TP, Niehaus JL, Meghan P, Roarty MP, Giesbrecht B, Miller MB. Neural correlates of detecting pretense: automatic engagement of the intentional stance under covert conditions. Journal of Cognitive Neuroscience. 2004;16:1805–17. doi: 10.1162/0898929042947892. [DOI] [PubMed] [Google Scholar]
- Gu X, Han S. Attention and reality constraints on the neutral processes of empathy for pain. Neuroimage. 2007;36:256–67. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Han S, Fan Y, Xu X, et al. Empathic neural responses to others' pain are modulated by emotional contexts. Human Brain Mapping. 2009;30:3227–37. doi: 10.1002/hbm.20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Current Opinion in Neurobiology. 2008;18:153–8. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain: an event-related fMRI study. Neuropsychologia. 2006a;44:752–61. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others: a window into the neural processes involved in empathy. NeuroImage. 2005;24:771–9. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Rainville P, Decety J. To what extent do we share the pain of others? Insight from the neural bases of pain empathy. Pain. 2006b;125:5–9. doi: 10.1016/j.pain.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kreuzbauer R, Chiu CY. The psycho-economics of money and social support. Psychological Inquiry. 2008;19:148–52. [Google Scholar]
- Knutson B, Fong GW, Bennett WM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage. 2003;18:263–72. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007a;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lamm C, Nusbaum H, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE. 2007b;12:e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Psychology. 2007;58:259–89. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Decety J, Ohnishi T, et al. Empathy and judging other's pain: an fMRI study of alexithymia. Cerebral Cortex. 2007;17:2223–34. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- Morrison I, Downing PE. Organization of felt and seen pain responses in anterior cingulate cortex. Neuroimage. 2007a;37:642–51. doi: 10.1016/j.neuroimage.2007.03.079. [DOI] [PubMed] [Google Scholar]
- Morrison I, Lloyd D, di Pellegrino G, Robets N. Vicarious responses to pain in anterior cingulate cortex: is empathy a multisensory issue? Cognitive & Affective Behavioural Neuroscience. 2004;4:270–8. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- Morrison I, Peelen MV, Downing PE. The sight of others' pain modulates motor processing in human cingulate cortex. Cerebral Cortex. 2007b;17:2214–22. doi: 10.1093/cercor/bhl129. [DOI] [PubMed] [Google Scholar]
- Overwalle FV. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30:829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FBM. Empathy: its ultimate and proximate bases. Behavioural and Brain Sciences. 2002;25:1–72. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Rameson LT, Lieberman MD. Empathy: a social cognitive neuroscience approach. Social and Personality Psychology Compass. 2009;3:94–110. [Google Scholar]
- Saarela MV, Hlushchuk Y, Williams AC, Schurmann M, Kalso E, Hari R. The compassionate brain: humans detect intensity of pain from another's face. Cerebral Cortex. 2007;17:230–7. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta J. Individual differences in reward responding: explain placebo-induced expectations and effects. Neuron. 2007;55:325–36. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not the sensory components of pain. Science. 2004;303:1157–61. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RH, Powell CAJ, Combs DJY, Schurtz DR. Exploring the when and why of schadenfreude. Social and Personality Psychology Compass. 2009;3:530–46. [Google Scholar]
- Sundie JM, Ward JC, Beal DJ, Chin WW, Geiger-Oneto S. Schadenfreude as a consumption-related emotion: feeling happiness about the downfall of another's product. Journal of Consumer Psychology. 2009;19:356–73. [Google Scholar]
- Valeriani M, Betti V, Le Pera D, et al. Seeing the pain of others while being in pain: a laser-evoked potentials study. Neuroimage. 2008;40:1419–28. doi: 10.1016/j.neuroimage.2007.12.056. [DOI] [PubMed] [Google Scholar]
- Vohs KD, Mead NL, Goode MR. Psychological consequences of money. Science. 2006;314:1154–6. doi: 10.1126/science.1132491. [DOI] [PubMed] [Google Scholar]
- Vollm BA, Taylor ANW, Richardson P, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–8. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and ‘mirror neuron’ functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–21. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Wrase J, Kahnt T, Schlagenhauf F, et al. Different neural systems adjust motor behavior in response to reward and punishment. NeuroImage. 2007;36:1253–62. doi: 10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. Journal of Neuroscience. 2009;29:8525–9. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XY, Gao DG. Social support and money as pain management mechanisms. Psychological Inquiry. 2008;19:127–44. [Google Scholar]
- Zhou XY, Vohs KD, Baumeister RF. The symbolic power of money: reminders of money alter social distress and physical pain. Psychological Science. 2009;20:700–6. doi: 10.1111/j.1467-9280.2009.02353.x. [DOI] [PubMed] [Google Scholar]