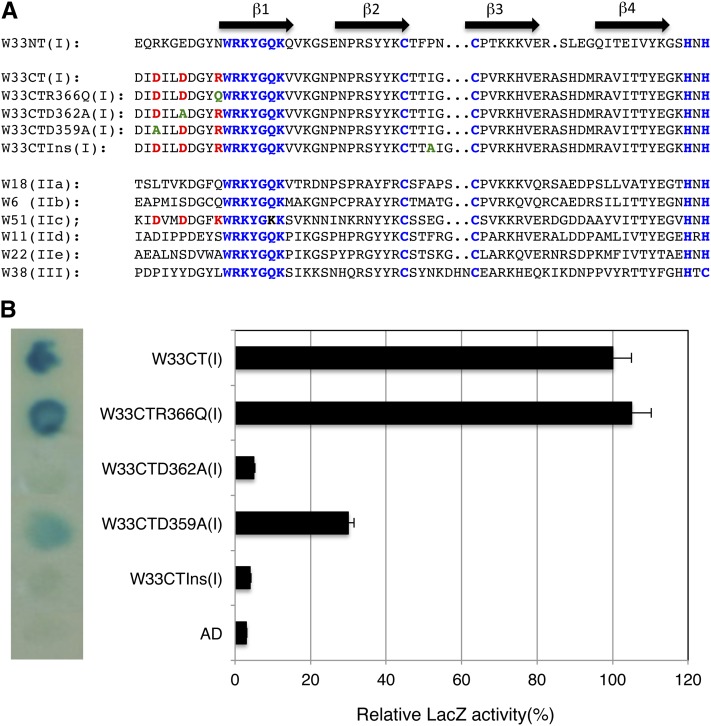
Figure 4.
Identification of critical amino acid residues of C-terminal WRKY domain of WRKY33 for interaction with VQ10. A, Sequence comparison of WRKY domains of WRKY33 (W33), WRKY18 (W18), WRKY6 (W6), WRKY51 (W51), WRKY11 (W11), WRKY22 (W22), WRKY38 (W38), which belong to group I, IIa, IIb, IIc, IId, IIe, and III WRKY proteins, respectively. Mutant C-terminal WRKY domains of WRKY33 (W33CT) with D395A, D362A, or R366Q substitution or with an Ala inserted between the two zinc-finger Cys residues (W33CTIns) are also shown. The WRKYGQK sequences and the Cys (C) and His (H) residues involved in zinc coordination are indicated in blue. The two Asp (D) residues and one basic residue in the region preceding the WRKYGQK sequence shared by the W33CT and W51 WRKY domains are in red. Resulted amino acid residues in the mutant W33CT proteins are in green. B, Yeast two-hybrid assays of interactions of VQ10 with wild-type and mutant forms of the C-terminal WRKY domains of WRKY33. pAD-VQ10 fusion vector was cotransformed with various pBD-W33CT constructs into yeast cells. Yeast transformants were analyzed for the LacZ reporter gene expression through assays of β-galactosidase activity on membrane using 5-bromo-4-chloro-3-indoly1-β-d-galactopyranoside (left section) or o-nitrophenyl-β-d-galactopyranose (right section) as substrate.