Table 1. Development of a Diastereo- and Enantioselective BisAMidine-Catalyzed Aryl Nitromethane Addition to Azomethinea.
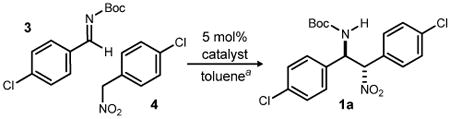 |
|||||||
|---|---|---|---|---|---|---|---|
| entry | BisAMidine | temp (°C) | TfOH (equiv) | dr | ee (%) | yieldb (%) | |
| 1 | 5c | −20 | 1 | 13:1 | 64 | 81 | 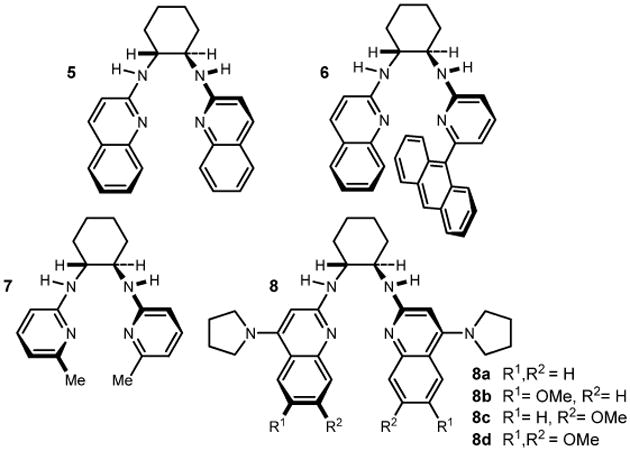 |
| 2 | 5c | −20 | 0 | 10:1 | 65 | 99 | |
| 3 | 6c | −20 | 1 | 7:1 | 80 | 87 | |
| 4 | 6c | −20 | 0 | 6:1 | 72 | 98 | |
| 5 | 7c | −20 | 1 | 5:1 | 58 | 95 | |
| 6 | 7c | −20 | 0 | 6:1 | 63 | 96 | |
| 7 | 8a | −78 | 1 | 5:1 | 85 | 99 | |
| 8 | 8a | −78 | 0 | 9:1 | 86 | 97 | |
| 9 | 8a | −20d | 0 | 6:1 | 83 | 99 | |
| 10 | 8b | −78 | 0 | 13:1 | 87 | 99 | |
| 11 | 8c | −78 | 0 | 16:1 | 89 | 99 | |
| 12 | 8d | −78 | 0 | 13:1 | 91 | 97 | |
Reactions employed 1 equivalent of α-bromo nitroalkane (0.2 M in THF), with NIS added as the final reagent at 25 °C.
Isolated yields.
2 Equivalents of imine used.
1.2 Equivalents of imine used.
