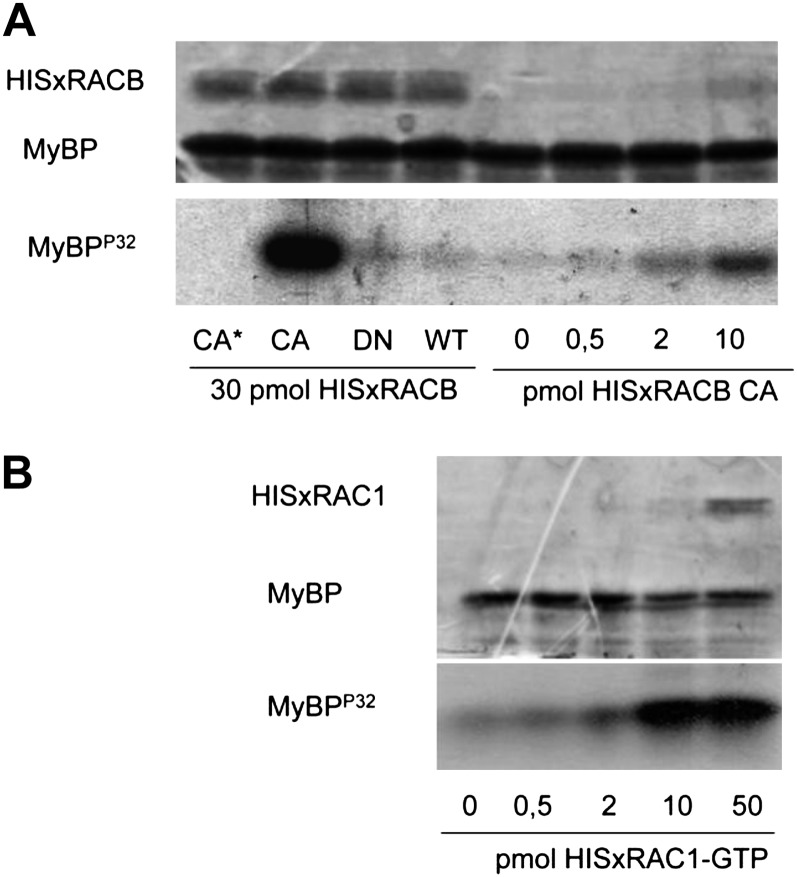
Figure 3.
Active HvRACB/HvRAC1 GTPases increase the activity of HvRBK1 in vitro. The in vitro myelin basic protein (MyBP) phosphorylating activity of HvRBK1 is shown in the presence of purified HIS-tagged barley HvRACB (A) and HvRAC1 (B) GTP-binding proteins. A, HISxHvRACB was added to the kinase reaction in CA, DN, and wild-type (WT) forms (30 pmol each). As a negative control (CA*), the same amount of the CA HvRACB GTPase was also added to a reaction mixture not containing the kinase protein (CA*). Moreover, kinase reactions were carried out in the presence of various CA HvRACB GTPase amounts (0–10 pmol). Protein loading is shown by Coomassie Brilliant Blue staining of the proteins in the kinase reaction after their separation in a polyacrylamide gel (top image), whereas kinase activity is demonstrated by the autoradiographic detection of radioactive MyBPP32 in the same gel (bottom image). B, HISxHvRAC1 was added to the kinase reaction at the indicated concentrations after loading the wild-type protein with GTP. The top image shows the result of the CBB staining and the bottom one the autoradiography of the gel used for the separation of proteins in the kinase reactions.