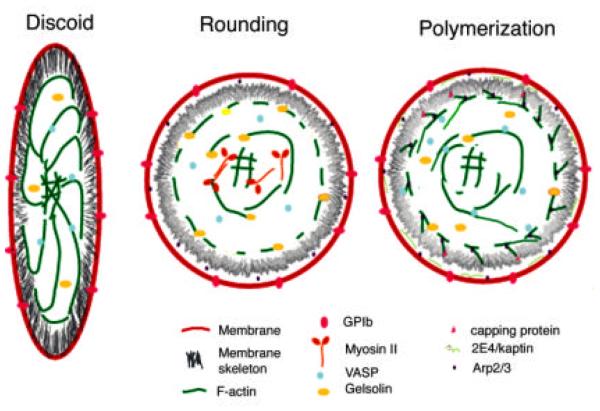
FIG 12.
Proposed model of actin reorganization in platelet activation and spreading. The discoid platelet has a trilaminar membrane shield composed of the outer membrane, the spectrin-ABP-rich inner membrane skeleton and a cage of closely aligned actin filaments that connect to the central core via radiating spokes. VASP bundles the filaments radiating from the central core. Most filaments are long and their barbed ends are capped. Monomer is sequestered by thymosin β4.
Upon activation, the platelet rounds and contracts, the membrane skeleton is dissociated, at least in part through proteolysis. Side-binding proteins such as VASP, are released from the radiating bundles and gelsolin is activated by a calcium influx to sever these filaments and the membrane-associated cage. Activation of myosin results in condensation of the radiating filaments, severed from their membrane anchors, into the central core and formation of the contractile ring.
Disassociation and severing are rapidly followed by rounds of polymerization of new actin from activated nucleators like Arp2/3 and 2E4/kaptin and from uncapping of severed barbed ends. Arp2/3 is activated at the membrane surface and on the sides of the severed filaments. Cofilin regulates filament length and recycles monomers that are recharged by profilin with ATP. Capping protein regulates which filaments are free to elongate, and hence the site of lamellipodia formation. VASP and other side-binding proteins restructure the new filaments into bundles. (See also color insert.)