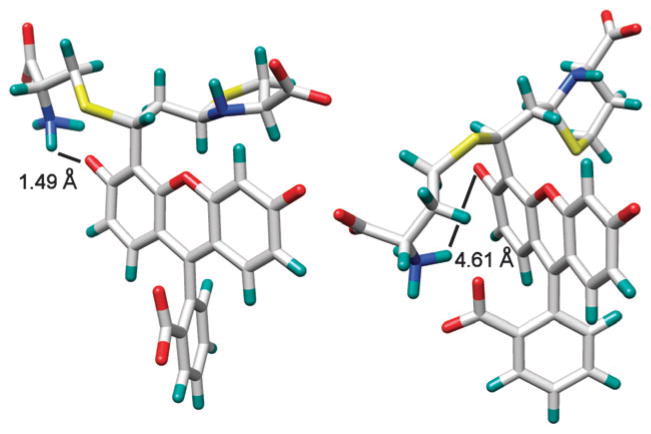
Fig. 3.
Energy-minimized structures of the reaction products of 1 with Cys (left) and Hcy (right) showing the distance between the NH3+ and the phenolate groups. The electrostatic interactions between NH3+ and the carboxylate and/or phenolate groups of 1 are influenced by increased ionic strength (addition of 1 M NaCl) resulting in a corresponding decrease in selectivity toward Cys (see Fig. S13–S15, ESI†).