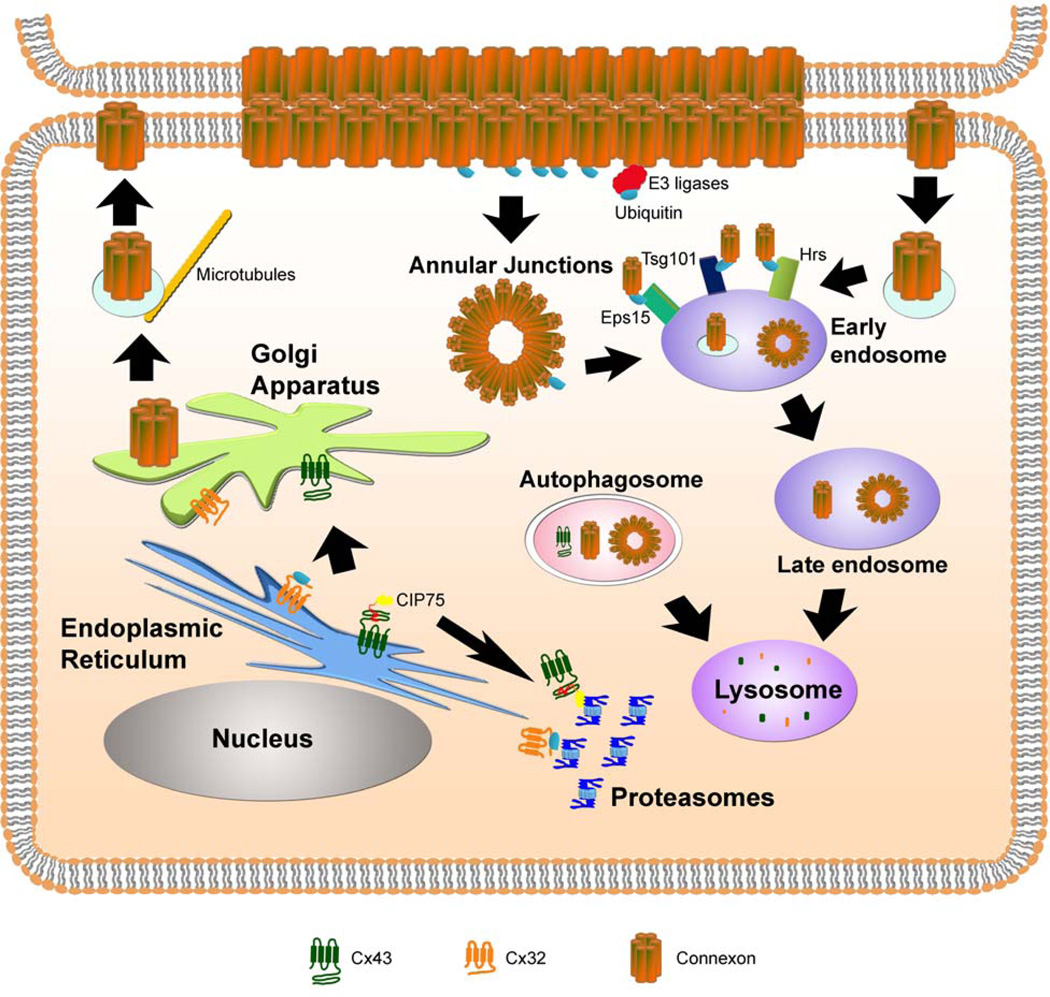
Figure 1. Regulation of the degradation of connexin proteins via proteasomal, lysosomal, and autophagic pathways.
During protein synthesis, connexins are co-translationally inserted into the ER membrane. Upon proper folding, connexins are subsequently trafficked to the Golgi apparatus and trans Golgi network where the individual proteins oligomerize into hexameric connexons. Misfolded connexins can be ubiquitinated and degraded by the proteasome through ERAD, as in the case of Cx32. Alternatively, non-ubiquitinated Cx43 interacts with the accessory factor, CIP75, which facilitates Cx43 ERAD. Connexons are trafficked to the plasma membrane on microtubules, where the hexamers dock with connexons on neighboring cells, across the intercellular space. Intact gap junctions are internalized as the double membrane annular junctions. Individual connexons may also undergo endocytosis. Annular junctions or internalized connexons may enter the endo-lysosomal pathway leading to the degradation of the connexin proteins. Ubiquitination of connexins by E3 ligases may occur either at the plasma membrane or after internalization from the membrane. The ubiquitin-interacting proteins (Eps15, Hrs, and Tsg101) may act to sort the ubiquitinated connexins through the endocytic pathway, until the connexins are ultimately degraded in the lysosome. Autophagy of connexins or connexons is another emerging mechanism for degradation.