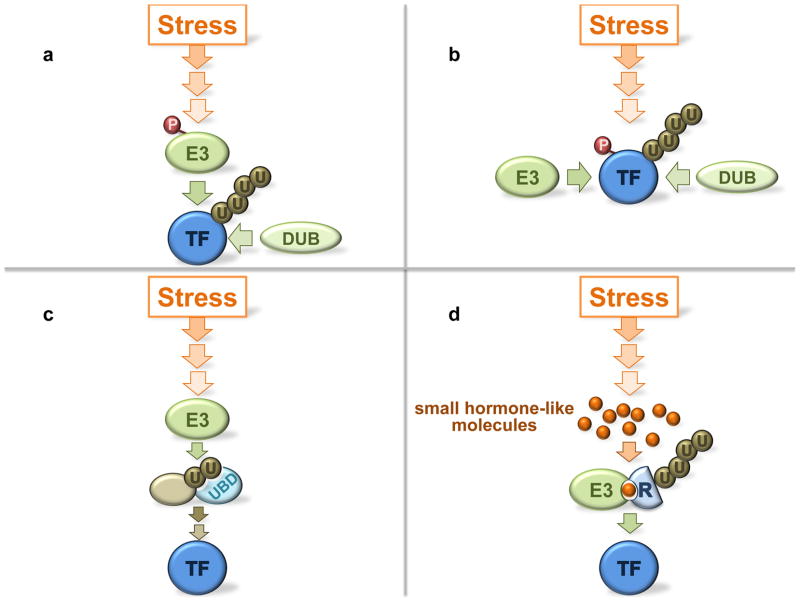
Fig. 2. Concepts of ubiquitin ligase involvement in stress signal transduction.
Four examples for how ubiquitin ligases transmit and control stress signals are depicted. (a) Ubiquitin ligases are part of the core signal flow and are directly controlled by upstream signaling components. Examples are Mdm2 phosphorylation or inhibition of Mdm2 by protein binding. Deubiquitylating enzymes (DUBs) may oppose ubiquitin ligase activity. (b) An E3 substrate is posttranslationally modified by the stress signal cascade and the modification prevents ubiquitin ligase binding, or stimulates binding. An example for the former is ATM mediated p53 phosphorylation. Protein modifications, particularly phosphorylation, as substrate recruitment signals are widespread and typical for SCF-type ligases. (c) Ubiquitylation of a signaling component creates a binding site for a downstream component that binds via an ubiquitin binding domain (UBD) and transmits the stress signal. Such ubiquitin signals can be monoubiquitin modifications or polyubiquitin chains, are typically non-proteolytic signals, and often involve specific polyubiquitin chain topologies such as K63-linked chains. Examples are abundant in DNA damage response pathways. (d) Plants frequently use small hormone like molecules for signaling and feature several cullin-RING ubiquitin ligases that recognize their substrates only in the presence of the hormone. The hormone acts as “molecular glue”, initiates substrate binding to the E3, and controls ubiquitylation and subsequent degradation. Examples are plant stress response pathways mediated by jasmonate. TF: transcription factor.