Figure 1.
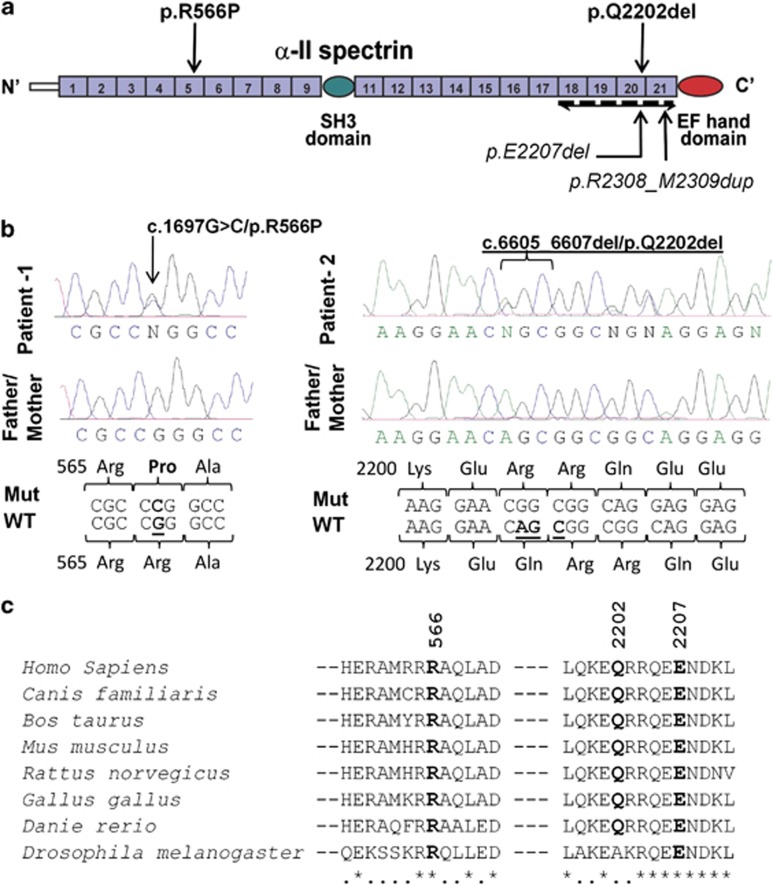
De novo mutations identified in SPTAN1. (a) Schematic representation of the SPTAN1 protein consisting of 22 domains, including 20-spectrin repeats, an SH3 domain, and an EF hand domains. The last four spectrin repeats required for α/β spectrin heterodimer associations are indicated (bidirectional arrow). Mutations identified in this study (p.R566P and p.Q2202del) and those by Saitsu et al4 (p.E2207del and p.R2308_M2309dup) are shown. (b) Chromatograms of the de novo p.R566P and p.Q2202del mutations (Mut) and wildtype (WT) SPTAN1 sequences. (c) Amino-acid conservation of the SPTAN1 residues affected with de novo mutations (p.R566P, p.Q2202del and p.E2207del). Amino-acid alignments were generated by homologene (NCBI) and the amino-acid sequences flanking the mutations are shown.