Abstract
Abnormalities of bone mineral metabolism and vascular calcification are prevalent in patients with kidney failure. Clinical management is based on biochemical targets, in particular parathyroid hormone (PTH) concentrations, but this has many limitations including high biological variation. A possible alternative is bone-specific alkaline phosphatase (ALP); therefore, we evaluated the biological variation of this marker in patients undergoing hemodialysis. Bone ALP was measured in non-fasting serum samples taken twice a week over a 6-week period in 22 stable hemodialysis patients and 12 healthy volunteers. The within-individual coefficients of variance were calculated and used to derive the critical difference required to be certain that an observed change was significant. The coefficient of variance for bone ALP was significantly higher in hemodialysis patients compared to healthy individuals. Seven samples were required to estimate the homeostatic set point of bone ALP, within 10%, in a hemodialysis patient. The concentration of serial bone ALP measurements would need to change by 36% between any two measurements before it can be considered a significant change. Since the biological variation of bone ALP is less than half that reported for PTH, our study provides further support for the use of bone ALP as an alternative marker of bone mineral metabolism in the setting of chronic kidney disease–mineral and bone disorder.
Keywords: bone, dialysis, hemodialysis, parathyroid hormone, renal osteodystrophy
Patients with kidney failure frequently develop chronic kidney disease–mineral and bone disorder (CKD-MBD).1 This term encompasses biochemical abnormalities such as secondary hyperparathyroidism along with alterations in bone morphology and extraskeletal (vascular) calcification.1 Studies have repeatedly demonstrated that CKD-MBD contributes to morbidity2 and mortality1, 3 in the CKD population. The gold standard for diagnosis of the bone component of CKD-MBD is bone histomorphometry. However, this is a costly, painful, and invasive procedure that is not routinely performed in clinical practice. Instead, clinical management focuses on biochemical targets,4, 5 especially plasma parathyroid hormone (PTH). The limitations of PTH as a marker in this respect are increasingly appreciated.6 Recently, we have demonstrated the high biological variability of PTH in patients on hemodialysis.7 Our data showed that in hemodialysis patients at least 26 specimens are required in order to estimate an individual's homeostatic set point within 10%. In addition, the concentration of PTH must differ by >72% between any two measurements before it can be considered a significant change. These data raise further concerns over the use of PTH and PTH target concentrations in CKD-MBD and emphasize the need for better markers.
A possible alternative is serum bone–specific alkaline phosphatase (bone ALP), a marker of bone turnover. However, no data concerning the biological variation of bone ALP in the hemodialysis population have been reported. The measurement of any laboratory analyte is subject to error or variation, which can be divided into three categories: preanalytical, analytical, and biological. If sources of preanalytical variation are minimized, then intraindividual biological variation (CVI) can be estimated by removing the contribution of analytical variation (CVA) from total variation (CVT).8 These numerical data can be used to evaluate the number of samples required to estimate an individual's homeostatic set point and calculate the critical difference, also known as the reference change value. This is the degree of change that must be observed in any measured substance before that change can be considered statistically significant, and therefore has obvious clinical importance in the monitoring of any patients with a chronic condition. The importance of understanding biological variation in nephrology is increasingly realized.9 The aims of this study were to establish: (i) the within-subject biological variation of bone ALP in hemodialysis patients, (ii) how many measurements are required to estimate a true homeostatic set point in any individual, (iii) the critical difference, and (iv) how these results compare with those we previously reported for PTH.
RESULTS
Clinical details of our study group have been reported previously but are reproduced in Table 1 for ease of reference. Among the hemodialysis patients, no changes were observed in weight, Kt/V, energy, and protein and mineral intakes at the beginning and end of the study (P>0.05). Median (range) serum total ALP activity in all of the samples included in the study was 76 (30–289) U/l, compared with 76 (30–288) U/l in our original report,7 confirming that there had been no loss of total ALP activity during frozen storage.
Table 1. Characteristics of study subjects.
| Healthy volunteers | Hemodialysis patients | |
|---|---|---|
| n | 12 | 22 |
| Age, yearsa | 38.5 (13.3), 24–61 | 67.7 (12.3), 42–90b |
| Female, n (%) | 8 (67%) | 11 (50%) |
| Weight, kga | NA | 78.2 (16.5), 53.5–113.0 |
| Dialysis adequacy (Kt/V)a | NA | 1.42 (0.31) |
| Daily energy intake (kcal/kg)a | NA | 21.4 (7.9), 10.0–38.0 |
| Daily protein intake (g/kg)a | NA | 0.80, 0.30–1.30 |
| Daily phosphate intake (mg)a | NA | 982 (259), 524–1482 |
| Daily calcium intake (mg)a | NA | 723 (201), 337–1186 |
| Cause of renal failure, n | NA | Small kidneys/unknown etiology (7), glomerulonephritis (4), membranous nephropathy (3), pyelonephritis (2), diabetes mellitus (2), renal vascular disease (2), IgA nephropathy (1), Wegener's granulomatosis (1) |
| Bone ALP concentration (μg/l)a | 13.3, 4.8–26.8 | 15.2, 7.8–85.0 |
| Total ALP activity (U/l)a | 56, 32–105 | 79, 43–275b |
| PTH concentration (ng/l)a | 43, 22–101 | 168, 18–978b |
Abbreviations: ALP, alkaline phosphatase; IgA, immunoglobulin A; NA, not applicable; PTH, parathyroid hormone.
Data obtained at the beginning of study.
Significantly (P<0.05) different from the value in the healthy controls.
Values for continuous variables are expressed as mean (s.d.), range (where given), or median, range. Further details may be found in Gardham et al.7
Cochran's test for outliers among duplicates identified two bone ALP measurements, both from the hemodialysis group (sample 5 from subject 9 and sample 11 from subject 19); these samples were excluded from statistical analysis. The CVs of these duplicates were the only ones above 10%. Cochran's test identified two samples that needed to be excluded from within-subject variance estimations (sample 6 from subject 7 and sample 3 from subject 25). Reed's test did not identify any outliers. Sample 11 from subject 13 and sample 2 from subject 26 were excluded because of suspected contamination (undetectable total ALP result). A number of individuals' observations displayed a significant trend over the length of the investigation (e.g., a consistent rise or fall in analyte concentration over time; Supplementary Table S1 and Figure S1 online). Analysis of variance performed both including and excluding the subjects showing trends yielded essentially the same estimates of CVA and CVI (Supplementary Table S2 online): consequently, the data presented in this paper include all subjects (i.e., those who both did and did not show trends in concentration over time).
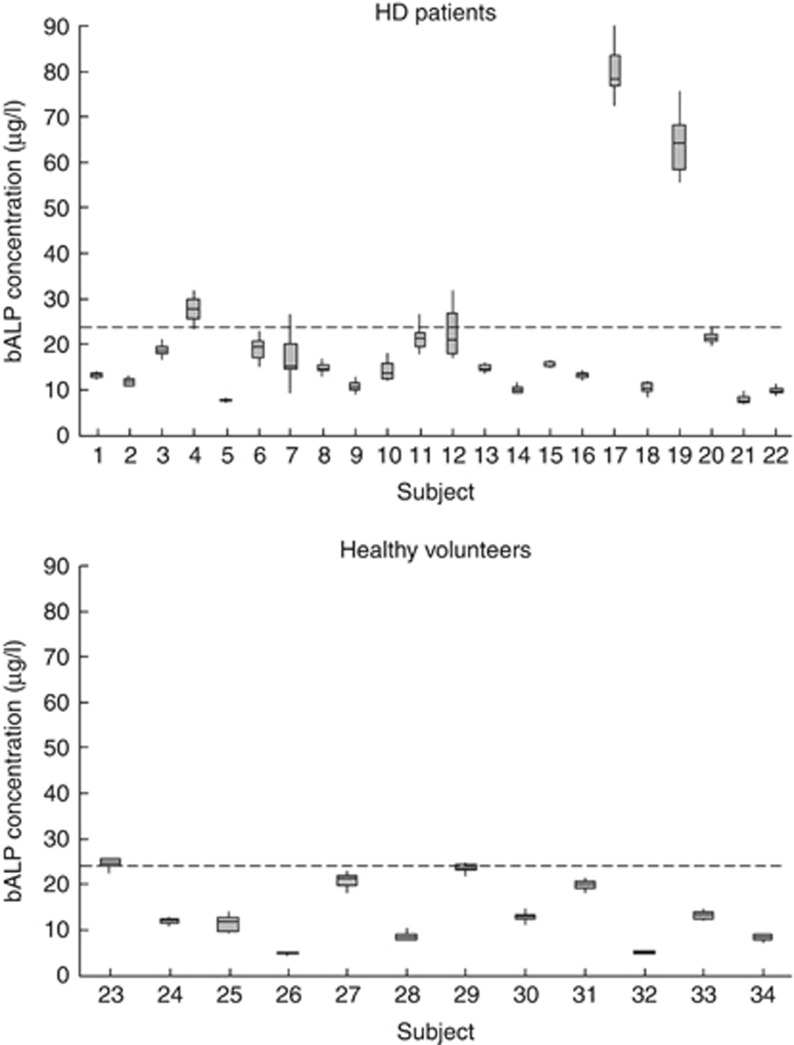
Figure 1 shows the individual median and range concentrations for bone ALP in all the study subjects. The CVI for bone ALP was significantly higher for the hemodialysis patients compared with the healthy volunteers (P<0.05), resulting in a greater number of specimens being required to estimate an individual's homeostatic set point and a higher critical difference (Table 2). No significant difference (P>0.05) in the CVI for bone ALP was observed between those patients who underwent dialysis in the morning (12.9%) and those who underwent dialysis in the afternoon (12.2%).
Figure 1.
A box plot showing median (horizontal line), upper, and lower quartile (large rectangle), as well as range (represented by the whiskers), for serum bone alkaline phosphatase (bALP) concentration for hemodialysis (HD) patients (subjects 1–22) and the healthy volunteers (subjects 23–34). The dashed lines represent the upper reference limit (22.4 μg/l) for the assay used. Note that study numbers are the same as in our previously published study,7 enabling direct comparison with parathyroid hormone (PTH) variability and concentrations within subjects.
Table 2. Estimation of variance components for healthy volunteers and hemodialysis patients including the critical difference and the number of specimens required to estimate the homeostatic set point of an individual (within ±10, ±20, and ±30% with a confidence of 95%).
|
Healthy volunteers |
Hemodialysis patients |
|||||
|---|---|---|---|---|---|---|
| Bone ALP | Total ALPa | PTHa | Bone ALP | Total ALPa | PTHa | |
| CVA | 3.0 | 1.2 | 3.5 | 3.4 | 0.9 | 3.6 |
| CVI | 7.7 | 5.8 | 19.2 | 12.5b | 9.9b | 25.6b |
| No. of specimens ±10% | 3 | 1 | 15 | 7 | 4 | 26 |
| No. of specimens ±20% | 1 | 1 | 4 | 2 | 1 | 6 |
| No. of specimens ±30% | 1 | 1 | 2 | 1 | 1 | 3 |
| Critical difference (%) | 23 | 16 | 54 | 36 | 27 | 72 |
Abbreviations: ALP, alkaline phosphatase; CVA, analytical variance; CVI, intraindividual variance; PTH, parathyroid hormone.
PTH and ALP data are same as published previously7 and are shown here for comparison.
Significantly different than the equivalent variance component in the healthy volunteers (P<0.05).
Bone ALP concentrations were correlated with total ALP activity irrespective of whether all subjects were considered together (rs 0.86) or whether the healthy control group (rs 0.94) and hemodialysis patients (rs 0.86) were considered separately (P<0.0001 in all three cases). There were no significant correlations (P>0.05) between either bone ALP or total ALP and PTH irrespective of whether controls and patients were considered separately or together.
DISCUSSION
Bone ALP is an 80-kDa glycoprotein found on the surface of osteoblasts. Its concentration generally reflects the rate of bone formation in skeletal tissue. As bone formation is coupled to bone resorption, bone ALP measurements provide an indication of overall bone metabolism. Bone ALP can be used as a marker of bone turnover in CKD-MBD patients.10, 11 In this study, we have described for the first time the biological variation of bone ALP in hemodiaylsis patients and compared it against that we previously described for PTH.7
The CVI for bone ALP that we observed among healthy subjects (7.7%) is similar to that reported by others (3.4 (ref. 12), 6.6 (ref. 13), 7.7 (ref. 14), 9.0% (ref. 15)). Differences between reports could, for example, reflect other studies that only included exclusively premenopausal12 or postmenopausal15 women.
Biological variation for bone ALP was higher among hemodialysis patients than healthy volunteers, as we previously observed for total ALP, calcium, phosphate, and PTH.7 We cannot exclude the possibility that this reflects the older age of our hemodialysis patients compared with the healthy volunteers. However, CKD-MBD is almost universal among patients with kidney failure, and it is perhaps not unexpected that these markers will show a significantly higher CVI in hemodialysis patients. Similar observations have been made for total ALP when comparing healthy controls (CVI 4.5%) with Pagetic patients (CVI 12.4%).12 The higher CVI in disease compared with health clearly affects the critical difference and number of specimens required to estimate an individual's homeostatic set point. As noted by others,16 variance components derived from healthy subjects cannot necessarily be extrapolated to pathological situations.
However, the CVI of bone ALP in hemodialysis patients (12.5%) was less than half of that previously described for PTH (25.6%), resulting in a lower number of specimens required to estimate an individual's homeostatic set point (7 vs. 26) and a lower critical difference (36% compared with 72% Table 2). In addition, the biological variation of bone ALP in dialysis patients appeared to be consistent throughout the day, whereas we previously found suggestive evidence that the CVI of PTH differed between the morning and afternoon.7
The lower biological variation of bone ALP compared with PTH represents a potentially important advantage of this marker in the monitoring and treatment of CKD-MBD. More reliance can thus be placed on results and on changes in concentration. In addition to higher biological variation, PTH is an imperfect marker of CKD-MBD for a variety of other reasons including the following: analyte instability;17 significant phlebotomy sample site variation;18 standardization-related assay variability;19 nonspecificity of assays, with which ∼50% of measured PTH is of a biologically inactive form;20 racial variation in the PTH–histology relationship;21 a strong relationship between PTH and body mass index22 and nutritional/inflammatory status;23 poor histological correlation to target ranges;24 and skeletal resistance.25 Furthermore, attainment of the recommended PTH target range has not been shown to improve the risk of death or cardiovascular events within the CKD population.26, 27 These issues raise significant concerns over the validity of specific target concentrations of PTH and the use of PTH in monitoring response to treatment.
There are many potential biomarkers of skeletal health and integrity, some of which are difficult to use and to interpret in kidney failure on account of renal function affecting their plasma concentrations. One of the best known, and best established, of these is the plasma activity of ALP, which is increased in osteitis fibrosa.28 High plasma ALP activity among hemodialysis patients is associated with increased risks of all-cause and cardiovascular mortality,29, 30, 31 and it has been shown to be more sensitive to the effects of vitamin D replacement than PTH.32, 33 However, the use of total ALP in this context has always been considered limited by its nonspecificity for bone disease, with only ∼50% of blood activity being attributable to bone ALP.
Specific immunoassays for bone ALP have been available for nearly 20 years, but their use has not become widespread in nephrology, probably reflecting perceived difficulties of measurement and the primary role assigned to PTH in the management of CKD-MBD. Bone ALP has a relatively long half-life of 1–2 days, and its plasma concentration depends only on its rate of release from the osteoblasts and on its hepatic degradation. Unlike PTH, bone ALP does not accumulate with progressive loss of GFR.34 Further, bone ALP has a good relationship with bone histology10, 11, 21 and bone mineral density35 and is also a good predictor, with DEXA-derived bone mineral density, of future fracture risk in dialysis patients.36 In addition, high concentrations of bone ALP are associated with increased cardiovascular and all-cause mortality in kidney failure patients.37 Mechanistically, there is evidence implicating bone ALP as a proponent of vascular calcification in CKD-MBD.38, 39, 40 This is supported by data showing a significant association between serum ALP activity and coronary artery calcification in hemodialysis patients, with serum ALP >120 U/l being a robust predictor of calcification.41 Hence, our data, together with other published literature, advocate the use of bone ALP as a potential alternative marker of CKD-MBD, providing a living biopsy of bone activity with additional potential value in the risk stratification of hemodialysis patients.
Our samples were stored for 2 years at −80°C before analysis. However, no loss in serum total ALP activity was seen during this time. As bone ALP represents a significant component of total ALP, it is a reasonable assumption that bone ALP was also stable under these storage conditions. The CVI, and consequently the critical difference, for total ALP was lower than that for bone ALP; there was a strong correlation between bone ALP and total ALP; as noted above, total ALP has strong relationships with outcome in patients with kidney failure; further, total ALP is easier and cheaper to measure in laboratories than bone ALP. It is possible that the value of total ALP as a marker of CKD-MBD should be reassessed. However, we feel that its nonspecificity for bone tissue will always remain an impediment to clinical acceptance in this situation. Further, bone ALP shows stronger relationships with histological changes than total ALP, and better sensitivity and specificity for both low- and high-turnover bone disease.10 Although bone ALP requires far fewer samples than PTH to establish a patient's homeostatic set point, it is still clear that a single measurement is insufficient.
The significance of the reduced critical difference for bone ALP compared with PTH is best appreciated by application to a clinical example. In the study of Fletcher et al.11, who used the forerunner of our assay, mean bone ALP concentrations of 44 μg/l were observed in dialysis patients with severe hyperparathyroidism (mean PTH 780 ng/l) compared with 10 μg/l among patients with adynamic bone disease (mean PTH 140 ng/l). Applying critical differences (Table 2) to the values lying exactly intermediate between these two groups (bone ALP 27 μg/l and PTH 460 ng/l), it can be seen that a 72% increase in PTH (just significant) will result in a concentration of 790 ng/l, in excess of the mean of the group with severe hyperparathyroidism, whereas a 72% decrease would result in a concentration below the mean of the group with adynamic bone disease (129 ng/l). This, in part, may explain the poor histological correlation of bone biopsy specimens to PTH target ranges.24 For bone ALP, the same manipulation would result in concentrations of 17 μg/l and 37 μg/l, above and below the adynamic and severely hyperparathyroid means, respectively. This illustrates how the better signal-to-noise ratio of bone ALP compared with PTH would enable clinicians to better interpret changes in concentrations in their patients.
The concentrations of bone ALP we observed in our hemodialysis patients (mean 20 μg/l) were only moderately elevated compared with the control group. Urena et al.10 observed mean bone ALP concentration of 64 μg/l among patients with osteitis fibrosa, and Fletcher et al.11 observed mean bone ALP concentrations of 44 μg/l in dialysis patients with severe hyperparathyroidism (mean PTH 780 ng/l). Two of our patients (subjects 17 and 19) had markedly increased, and one (subject 4) marginally increased, median bone ALP concentrations: these subjects also had high PTH concentrations (>400 ng/l). Bone ALP concentrations were not significantly increased in the remaining patients. Overall, this would be consistent with an interpretation that the CKD-MBD among our cohort was at the milder end of the spectrum, possibly reflecting a shift in the phenotype of CKD-MBD away from high-turnover bone disease, as noted by others,42 compared with these earlier studies. However, the majority of the patients had PTH concentrations in excess of the upper limit of normal. In the absence of bone biopsy data, one cannot draw strong conclusions in this regard, but it is tempting to speculate that the high PTH concentrations may not be reflecting increased bone turnover in all of these individuals, because of some of the problems alluded to above. As we have noted previously,7 our cohort is fairly typical of hemodialysis cohorts in the UK at the present time, and we therefore feel our conclusions are generally applicable to this population.
In conclusion, we propose that the relatively low biological variability of bone ALP provides further suggestive evidence that it could be a better alternative to PTH measurement in the management of CKD-MBD in the adult hemodialysis population. Whichever marker is used, it is clear that clinicians cannot rely on single measurements assessed against rigid and somewhat arbitrary management targets, but instead must look serially for trends in patient results.
MATERIALS AND METHODS
Twenty-two patients receiving three-times-weekly hemodialysis for renal failure at the Kent Kidney Care Centre were recruited in 2008, with their informed consent. Ethical approval for this study was granted from the East Kent Research Ethics Committee (reference number 08/H1103/45). Complete details of the patients included in the study and of the exclusion criteria applied have been published previously.7 Briefly, all patients had been on hemodialysis for a minimum of 3 months, and their pharmacologic prescription and dietary intake remained consistent throughout the study period. Most were receiving 1α-hydroxycholecalciferol (Alfacalcidol) and calcium carbonate (Calcichew). To standardize preanalytical variables, only patients who underwent dialysis on Monday, Wednesday, and Friday were recruited. Twelve volunteer staff members who had a negative proteinuria screening test (Multistix 10 SG, Siemens Healthcare, Surrey, UK) and estimated GFR ⩾60 ml/min per 1.73 m2 (ref. 43) also participated in this study.
Non-fasting blood samples were collected before dialysis from the vascular access (arteriovenous fistula) of the patients before their dialysis sessions on Wednesday and Friday for 6 weeks (i.e., 12 samples per patient). Blood was collected from patients who underwent dialysis in the morning (n=13) between 0800 and 1000 hours, and from patients who underwent dialysis in the afternoon (n=9) between 1300 and 1500 hours. Concurrently, samples were collected from the 12 healthy volunteers on Wednesday and Friday (between 0930 and 1130 hours). Blood was collected in serum separator tubes (Becton-Dickinson Vacutainer Systems, Oxford, UK), and serum was separated within 4 h of venepuncture and stored at −80°C for 22 months until assayed. Before analysis, samples were thawed at room temperature for 48 h to enable reactivation of ALPs and then mixed by inversion. For analyte measurement, each sample was measured in duplicate in random order. A single operator performed all analyses using a single instrument.
Serum total ALP was measured on an Abbott Architect c8000 automated system (Abbott Laboratories, Abbott Park, IL) at 37°C using essentially the method described by the International Federation of Clinical Chemistry.44 The adult reference range for this assay is 42–128 U/l.45 Serum concentrations of bone ALP were determined using a solid-phase, monoclonal antibody immunoenzymetric assay (Ostase® BAP, Immunodiagnostic Systems, Tyne & Wear, UK). The upper limit of the reference range for this assay is 22.4 μg/l (manufacturer's product information).
Data analyses
All calculations and statistical analyses were carried out with Minitab (Coventry, UK) and Analyse-It™ (Analyse-It Software, Leeds, UK). Comparisons between dietary intakes, weight, and Kt/V at the start and end of the study were made using paired t-test or Wilcoxon match-pairs signed ranks test as appropriate. Comparisons between total ALP, bone ALP, and PTH concentrations at the start of the study between healthy controls and patients were made using the Mann–Whitney U-test. Cochran's criterion test was used to identify outliers among duplicate measurements and among within-subject variances, and the Reed test to eliminate mean outlying values.8 We tested individuals' data for normality using the Shapiro–Wilk test: approximately one-third of the data sets were not normally distributed. All variance estimates were therefore derived using natural logarithmic–transformed data after exclusion of outliers as recommended,8 and then back-transforming the data. Analytical (SDA2) and intraindividual variances (SDI2) were calculated by nested analysis of variance according to the methods of Fraser and Harris.8 Coefficients of variation (CVA and CVI) were calculated as CV%=SD/mean analyte concentration × 100. The F-test was used to compare: (i) CVI obtained for the healthy volunteers and the hemodialysis patients and (ii) CVI among patients who underwent dialysis in the morning with those who underwent dialysis in the afternoon. A P-value of <0.05 was set for statistical significance. As the aim of the study was to establish biological variation under steady-state conditions, exploratory analysis was conducted using linear regression analysis in order to discover whether the natural logarithmic data for bone ALP concentrations followed a trend over the 6-week period (e.g., a consistent rise or fall in concentrations). Analysis of variance analysis to determine CVI was then performed both including and excluding the subjects showing trends. Subjects with T values greater than +2 or less than −2 had measurements that showed a significant trend. The critical difference, which is the difference that must be exceeded between two sequential results for a significant change to have occurred in either direction at P<0.05, was derived by applying the standard equation [2.77 × (SDA2+SDI2)1/2]. The number of specimens required to estimate the homeostatic set point of an individual to within ±10% with 95% confidence was also calculated, and this analysis was adjusted to consider ±20 and ±30% limits. Spearman's correlation was used to assess relationships between bone ALP and both total ALP and PTH: in all cases the mean value for each subject was used.
Acknowledgments
We are grateful to the patients and healthy volunteers for agreeing to participate in this study. This study would not have been possible without the support of the nursing staff of the Kent Kidney Care Centre and phlebotomy and laboratory staff of the Department of Laboratory Medicine. Statistical advice was provided by Drs Alexa Laurence and Eryl Bassett of the Institute of Mathematics, Statistics and Actuarial Science at the University of Kent. Part of the funding for this project was provided by the Association for Clinical Biochemistry.
All the authors declared no competing interests.
Footnotes
SUPPLEMENTARY MATERIAL
Table S1. Subjects demonstrating significant data trends during the study.
Table S2. Estimation of variance components for bone alkaline phosphatase (bone ALP) in healthy volunteers and hemodialysis patients.
Figure S1. Serum bone alkaline phosphatase (bALP) concentration over the six-week period for (A) hemodialysis patients (subjects 1-22) and (B) healthy volunteers (subjects 23–34).
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
References
- Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- Danese MD, Kim J, Doan QV, et al. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis. 2006;47:149–156. doi: 10.1053/j.ajkd.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Kalpakian MA, Mehrotra R. Vascular calcification and disordered mineral metabolism in dialysis patients. Semin Dial. 2007;20:139–143. doi: 10.1111/j.1525-139X.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- United Kingdom Renal Association Clinical practice guidelines, 4th edn.2007
- Kidney Disease Improving Global Outcomes Clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease related mineral and bone disorders (CKD-MBD)2008
- Drueke TB. Is parathyroid hormone measurement useful for the diagnosis of renal bone disease. Kidney Int. 2008;73:674–676. doi: 10.1038/sj.ki.5002800. [DOI] [PubMed] [Google Scholar]
- Gardham C, Stevens PE, Delaney MP, et al. Variability of parathyroid hormone and other markers of bone mineral metabolism in patients receiving hemodialysis. Clin J Am Soc Nephrol. 2010;5:1261–1267. doi: 10.2215/CJN.09471209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci. 1989;27:409–437. doi: 10.3109/10408368909106595. [DOI] [PubMed] [Google Scholar]
- Houillier P, Coste J, Froissart M. How many measurements to make a decision. Clin J Am Soc Nephrol. 2010;5:1161–1162. doi: 10.2215/CJN.04440510. [DOI] [PubMed] [Google Scholar]
- Urena P, Hruby M, Ferreira A, et al. Plasma total vs. bone alkaline phosphatase as markers of bone turnover in hemodialysis patients. J Am Soc Nephrol. 1996;7:506–512. doi: 10.1681/ASN.V73506. [DOI] [PubMed] [Google Scholar]
- Fletcher S, Jones RG, Rayner HC, et al. Assessment of renal osteodystrophy in dialysis patients: use of bone alkaline phosphatase, bone mineral density and parathyroid ultrasound in comparison with bone histology. Nephron. 1997;75:412–419. doi: 10.1159/000189578. [DOI] [PubMed] [Google Scholar]
- Alvarez L, Ricos C, Peris P, et al. Components of biological variation of biochemical markers of bone turnover in Paget′s bone disease. Bone. 2000;26:571–576. doi: 10.1016/s8756-3282(00)00279-9. [DOI] [PubMed] [Google Scholar]
- Panteghini M, Pagani F. Biological variation in bone-derived biochemical markers in serum. Scand J Clin Lab Invest. 1995;55:609–616. doi: 10.3109/00365519509110260. [DOI] [PubMed] [Google Scholar]
- Scariano JK, Garry PJ, Montoya GD, et al. Critical differences in the serial measurement of three biochemical markers of bone turnover in the sera of pre- and postmenopausal women. Clin Biochem. 2001;34:639–644. doi: 10.1016/s0009-9120(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Hannon R, Blumsohn A, Naylor K, et al. Response of biochemical markers of bone turnover to hormone replacement therapy: impact of biological variability. J Bone Miner Res. 1998;13:1124–1133. doi: 10.1359/jbmr.1998.13.7.1124. [DOI] [PubMed] [Google Scholar]
- Ricos C, Iglesias N, Garcia-Lario JV, et al. Within-subject biological variation in disease: collated data and clinical consequences. Ann Clin Biochem. 2007;44:343–352. doi: 10.1258/000456307780945633. [DOI] [PubMed] [Google Scholar]
- Teal TK, Reed M, Stevens PE, et al. Stability of parathyroid hormone ex vivo in haemodialysis patients. Ann Clin Biochem. 2003;40:191–193. doi: 10.1258/000456303763046175. [DOI] [PubMed] [Google Scholar]
- Vulpio C, Bossola M, Speranza D, et al. Influence of blood sampling site on intact parathyroid hormone concentrations in hemodialysis patients. Clin Chem. 2010;56:489–490. doi: 10.1373/clinchem.2009.136754. [DOI] [PubMed] [Google Scholar]
- Lamb EJ, Vickery S, Ellis AR. Parathyroid hormone, kidney disease, evidence and guidelines. Ann Clin Biochem. 2007;44:1–4. doi: 10.1258/000456307779596039. [DOI] [PubMed] [Google Scholar]
- Brossard JH, Cloutier M, Roy L, et al. Accumulation of a non-(1–84) molecular form of parathyroid hormone (PTH) detected by intact PTH assay in renal failure: importance in the interpretation of PTH values. J Clin Endocrinol Metab. 1996;81:3923–3929. doi: 10.1210/jcem.81.11.8923839. [DOI] [PubMed] [Google Scholar]
- Moore C, Yee J, Malluche H, et al. Relationship between bone histology and markers of bone and mineral metabolism in African-American hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1484–1493. doi: 10.2215/CJN.01770408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler C, Grootendorst DC, Boeschoten EW, et al. Changes in parathyroid hormone, body mass index and the association with mortality in dialysis patients. Nephrol Dial Transplant. 2011;26:1340–1346. doi: 10.1093/ndt/gfq541. [DOI] [PubMed] [Google Scholar]
- Drechsler C, Krane V, Grootendorst DC, et al. The association between parathyroid hormone and mortality in dialysis patients is modified by wasting. Nephrol Dial Transplant. 2009;24:3151–3157. doi: 10.1093/ndt/gfp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto FC, Barreto DV, Moyses RM, et al. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008;73:771–777. doi: 10.1038/sj.ki.5002769. [DOI] [PubMed] [Google Scholar]
- Ritz E, Malluche HH, Krempien B, et al. Pathogenesis of renal osteodystrophy: roles of phosphate and skeletal resistance to PTH. Adv Exp Med Biol. 1978;103:423–436. doi: 10.1007/978-1-4684-7758-0_44. [DOI] [PubMed] [Google Scholar]
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119–1127. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- Tangri N, Wagner M, Griffith JL, et al. Effect of bone mineral guideline target achievement on mortality in incident dialysis patients: an analysis of the United Kingdom Renal Registry. Am J Kidney Dis. 2011;57:415–421. doi: 10.1053/j.ajkd.2010.08.037. [DOI] [PubMed] [Google Scholar]
- Duarte ME, Peixoto AL, Pacheco AS, et al. The spectrum of bone disease in 200 chronic hemodialysis patients: a correlation between clinical, biochemical and histological findings. Sao Paulo Med J. 1998;116:1790–1797. doi: 10.1590/s1516-31801998000500002. [DOI] [PubMed] [Google Scholar]
- Regidor DL, Kovesdy CP, Mehrotra R, et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddhu S, Baird B, Ma X, et al. Serum alkaline phosphatase and mortality in hemodialysis patients. Clin Nephrol. 2010;74:91–96. doi: 10.5414/cnp74091. [DOI] [PubMed] [Google Scholar]
- Blayney MJ, Pisoni RL, Bragg-Gresham JL, et al. High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int. 2008;74:655–663. doi: 10.1038/ki.2008.248. [DOI] [PubMed] [Google Scholar]
- Palmer SC, McGregor DO, Macaskill P, et al. Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med. 2007;147:840–853. doi: 10.7326/0003-4819-147-12-200712180-00004. [DOI] [PubMed] [Google Scholar]
- Tanzy ME, Camacho PM. The effect of Vitamin D therapy on bone turnover markers in postmenopausal women with osteoporosis and osteopenia. Endocr Pract. 2011;17:873–879. doi: 10.4158/EP10339.OR. [DOI] [PubMed] [Google Scholar]
- Urena P, De Vernejoul MC. Circulating biochemical markers of bone remodeling in uremic patients. Kidney Int. 1999;55:2141–2156. doi: 10.1046/j.1523-1755.1999.00461.x. [DOI] [PubMed] [Google Scholar]
- Manghat P, Fraser WD, Wierzbicki AS, et al. Fibroblast growth factor-23 is associated with C-reactive protein, serum phosphate and bone mineral density in chronic kidney disease. Osteoporos Int. 2009;21:1853–1861. doi: 10.1007/s00198-009-1142-4. [DOI] [PubMed] [Google Scholar]
- Iimori S, Mori Y, Akita W, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—a single-center cohort study. Nephrol Dial Transplant. 2012;27:345–351. doi: 10.1093/ndt/gfr317. [DOI] [PubMed] [Google Scholar]
- Drechsler C, Verduijn M, Pilz S, et al. Bone alkaline phosphatase and mortality in dialysis patients. Clin J Am Soc Nephrol. 2011;6:1752–1759. doi: 10.2215/CJN.10091110. [DOI] [PubMed] [Google Scholar]
- Iba K, Takada J, Yamashita T. The serum level of bone-specific alkaline phosphatase activity is associated with aortic calcification in osteoporosis patients. J Bone Miner Metab. 2004;22:594–596. doi: 10.1007/s00774-004-0528-9. [DOI] [PubMed] [Google Scholar]
- Lomashvili K, Garg P, O'Neill WC. Chemical and hormonal determinants of vascular calcification in vitro. Kidney Int. 2006;69:1464–1470. doi: 10.1038/sj.ki.5000297. [DOI] [PubMed] [Google Scholar]
- Shioi A, Katagi M, Okuno Y, et al. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ Res. 2002;91:9–16. doi: 10.1161/01.res.0000026421.61398.f2. [DOI] [PubMed] [Google Scholar]
- Shantouf R, Kovesdy CP, Kim Y, et al. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1106–1114. doi: 10.2215/CJN.06091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazao JM, Martins P. Adynamic bone disease: clinical and therapeutic implications. Curr Opin Nephrol Hypertens. 2009;18:303–307. doi: 10.1097/MNH.0b013e32832c4df0. [DOI] [PubMed] [Google Scholar]
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- Tietz NW, Rinker AD, Shaw LM. IFCC methods for the measurement of catalytic concentration of enzymes Part 5. IFCC method for alkaline phosphatase (orthophosphoric-monoester phosphohydrolase, alkaline optimum, EC 3.1.3.1) J Clin Chem Clin Biochem. 1983;21:731–748. [PubMed] [Google Scholar]
- Tietz NW, Shuey DF. Reference intervals for alkaline phosphatase activity determined by the IFCC and AACC reference methods. Clin Chem. 1986;32:1593–1594. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.