There are approximately 8,500 new diagnoses of Hodgkin's lymphoma (HL) each year in the United States[1]. Patients who do not respond to initial therapy or who relapse after initial response are rarely cured with additional conventional chemotherapy alone. Two randomized trials have compared salvage chemotherapy to high-dose chemotherapy with autologous stem cell transplant (ASCT) with both showing a significant benefit in disease-free survival favoring ASCT[2,3]. ACST has therefore become the standard of care for patients with chemosensitive relapsed or refractory HL.
No single high-dose combination preparative regimen for ASCT has proven superior to another in patients with lymphoma, and therefore the choice is usually based on institutional experience and/or toxicity profile. Busulfan and cyclophosphamide, with or without etoposide, is well described as a preparative regimen for myeloablative allogeneic stem cell transplantation, and busulfan, cyclophsophamide, and etoposide (BuCyE) is a standard ASCT preparative regimen for patients with non-Hodgkin lymphoma[4] and HL[5,6]. We recently reported our experience with busulfan and cyclophosphamide (Bu/Cy) in patients with NHL, and showed comparable efficacy and toxicity to other high-dose chemotherapy regimens[7]. However, an experience of Bu/Cy alone has not yet been published for a series limited to patients with HL. Here, we describe our institution's experience with Bu/Cy-ASCT in patients with HL to present an additional option for high-dose chemotherapy in this patient population.
Between 1995 and 2008, 19 patients with chemosensitive relapsed or refractory Hodgkin lymphoma received Bu/Cy followed by autologous stem cell transplantation at the Massachusetts General Hospital Cancer Center (MGHCC). After Institutional Review Board approval, data was collected by retrospective review of electronic medical records. ASCT eligibility and supportive care were as described previously[7]. Overall survival was defined as the time from transplantation until death from any cause. Progression-free survival was defined as the time from transplantation until lymphoma relapse or death from any cause. Seven days prior to transplant, patients received either 1 mg/kg oral busulfan (5 of 19 patients) or, after December 2000, intravenous busulfan at 0.8 mg/kg, every 6 hours for 4 days for a total of 14 or 16 doses, with intravenous cyclophosphamide 60 mg/kg daily on days -3 and -2. Patients older than 50 years received 14 doses of busulfan while patients younger than 50 received 16 doses. Cyclophosphamide and oral busulfan were dosed at the average of ideal and actual body weight for patients >20% above ideal body weight. Intravenous busulfan dosing was based on either actual or ideal body weight, whichever was less. On day 0, patients received a minimum dose of 2×106 CD34+ cells/kg as an infusion of mobilized autologous peripheral blood stem cells (with bone marrow in two patients). Patients were hospitalized until neutrophil engraftment as defined by an ANC ≥500/μl on two consecutive days. Platelet engraftment was defined by an unsupported platelet count ≥50,000/μl on two consecutive occasions.
Clinical characteristics and toxicity data are displayed in Table I. There were no instances of autologous graft failure. Ten patients had grade 1-2 mucositis during their ASCT hospitalization. Three patients experienced documented bacterial infections (coagulase-negative Staphlococcus bacteremia in two patients, and acute appendicitis 45 days post-transplant in one), one of whom also had a fungal infection (Candida parapsilosis fungemia). There was one case of localized varicella zoster reactivation during the transplant admission.
Table I.
Patient characteristics and transplantation outcomes
| n (%) | |
|---|---|
| Total patients | 19 |
| Age (years) [median (range)] | 39 (22-79) |
| Male | 11 (58) |
| HIV positive | 2 (11) |
| Prior chemotherapy regimens [median (range)] | 2 (1-4) |
| Prior radiotherapy | 10 (53) |
| Duration of first response (months) [median(range)] | 12 (0-96) |
| Extranodal disease at relapse/progression | 12 (63) |
| Stage at relapse/progression | |
| IIA | 6 (32) |
| IIIA | 1 (5) |
| IVA | 7 (37) |
| IVB | 5 (26) |
| Status at ASCT | |
| Chemosensitive | 19 (100) |
| PR1 | 5 (26) |
| CR2 | 6 (32) |
| PR2/PR3 | 8 (42) |
| Days to neutrophil engraftment, median (range) | 10 (8-14) |
| Days to platelet engraftment, median (range) | 13 (9-123) |
| Hospital stay (days) [median (range)] | 19 (16-81) |
| 100-day TRM | 1 (5) |
| Infection, n (%) | |
| Bacterial | 3 (16) |
| Fungal | 1 (5) |
| Viral | 1 (5) |
| VOD | 1 (5) |
| Cardiac toxicity, n (%) | 1 (5) |
| CNS toxicity, n (%) | 1 (5) |
| Pulmonary toxicity, n (%) | 4 (21) |
| Secondary MDS/AML | 2 (11) |
HIV, human immunodeficiency virus; ASCT, autologous stem cell transplantation; CR or PR, complete remission or partial remission (as determined by PET or gallium, and CT scans prior to ASCT); VOD, veno-occlusive disease of the liver; CNS, central nervous system; TRM, treatment-related mortality; MDS, myelodysplastic syndrome; AML, acute myeloid leukemia
Transplant-related toxicities are summarized below. There was one case of hepatic veno-occlusive disease (VOD) in a 38 year-old woman with relapsed stage IIB HL. Thirteen days post-transplant she developed VOD, requiring intensive care unit care for multiorgan system dysfunction, but improved after treatment with defibrotide and ultimately survived to hospital discharge. There was one case of acute cardiac toxicity in a 62 year-old woman who developed transient congestive heart failure. This was postulated to be due to cyclophosphamide cardiotoxicity, which resolved to baseline cardiac function with medical management. There was one case of neurotoxicity in a 39 year-old female who received oral busulfan, and who had been on phenytoin prior to transplant for a seizure disorder. She experienced a generalized tonic-clonic seizure on her third day of conditioning despite having a phenytoin level in the therapeutic range. The final two doses of busulfan (of planned 16) were held and no further seizure activity occurred.
Four patients experienced pulmonary complications after ASCT. Three patients developed shortness of breath and decreased DLCO on pulmonary function testing consistent with pneumonitis, which resolved over several months with conservative therapy. The fourth patient was a 23 year-old male who died 88 days post-transplant due to pneumonitis-related complications. He had been treated with ABVD and mediastinal radiation for stage IIB HL one year prior to transplant, and subsequent salvage ICE chemotherapy at relapse. Pre-transplant testing revealed asymptomatic interstitial lung disease possibly related to prior chemotherapy and radiation, evidenced by a chest CT and pulmonary function tests. He presented 57 days post-transplant with severe shortness of breath and hypoxemia requiring intubation and mechanical ventilation. Lung wedge biopsy showed diffuse alveolar damage and early fibrosis. Extensive infectious studies and evaluation by bronchoscopy were negative. Despite high-dose steroid treatment, he developed right-sided heart failure and died 88 days post-transplant.
There were two cases of secondary myelodysplastic syndrome (MDS) after ASCT. Both occurred in patients who received oral busulfan as part of their conditioning regimen. Both patients were then treated with an allogeneic non-myeloablative matched related donor stem cell transplant. One patient died of infectious complications six months after allogeneic transplant. The other patient had recurrence with frank AML three months post allogeneic transplant and died of relapsed leukemia and infectious complications.
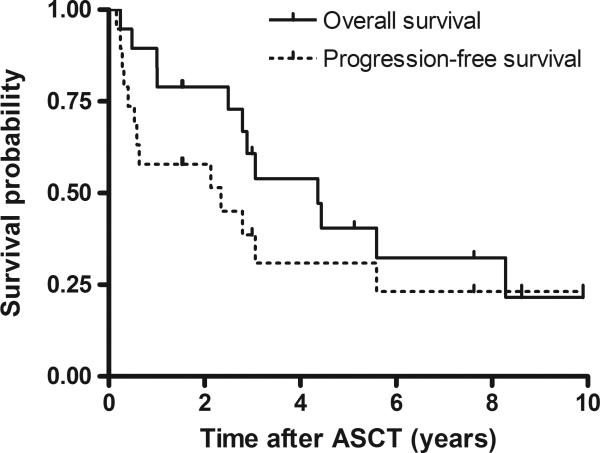
The median duration of follow-up for survivors was 5.1 years (range 1.5-9.9 years). Figure 1 shows Kaplan-Meier probability curves for overall (OS) and progression-free survival (PFS). At 3 years, progression-free survival was 39% (95% confidence interval [CI] = 15% to 62%), and overall survival was 61% (95% CI = 38% to 84%). At 5 years, progression-free survival was 31% (95% CI = 8% to 54%), and overall survival was 41% (95% CI = 16% to 65%). Twelve patients in the cohort had died at the time of data analysis. Eight patients died of relapsed lymphoma, two of secondary MDS/AML, one of therapy-related pneumonitis as described above, and one due to a cardiovascular event in a patient who was in sustained remission five years after ASCT.
Figure 1.
Overall and progression-free survival for all patients
While Bu/Cy conditioning has been reported in prior ASCT studies for patients with non-Hodgkin lymphoma[7] and multiple myeloma[8], to our knowledge, this is the first study to describe its use in a cohort of patients with HL. The outcomes reported in prior published studies of ASCT for relapsed or refractory HL have been summarized recently[9,10]. Five year PFS ranged from 40-49% and OS from 48-57%. Survival in our cohort may be inferior by comparison, although our patient population was not matched with prior studies with respect to age, comorbidities, and prior therapy. Furthermore, the small number of patients reported here precludes rigorous statistical comparison. Additionally, our cohort has a relatively high proportion of patients with established poor prognostic factors for survival after ASCT (reviewed in [9]), including stage IV and extranodal disease at relapse, and inclusion of five patients who did not reach a complete remission to initial chemotherapy.
Nonetheless, our experience suggests that Bu/Cy may not be as active against HL as other ASCT preparative chemotherapies, and therefore at our institution we reserve Bu/Cy as an alternative conditioning regimen. We previously described the use of Bu/Cy in elderly patients with NHL (above the age of 60), and noted favorable efficacy and toxicity outcomes[11]. Hence, our routine is to use Bu/Cy in lymphoma patients older than 70, or in those who have significant comorbidities, while higher intensity regimens such as CBV (cyclophosphamide, BCNU, and etoposide) and BuCyE are our institutional standards for younger patients with lymphoma undergoing ASCT.
When given as a component of conditioning chemotherapy for transplantation, intravenous busulfan is less toxic than when administered orally, likely due to improved pharmacokinetic reproducibility with intravenous dosing[12]. We find it notable that the two cases of secondary MDS, the case of VOD, and the case of neurotoxicity all occurred in patients who received oral busulfan. Furthermore, in two prior studies of patients with NHL who received a BuCyE regimen for ASCT using either oral or intravenous busulfan, there was a survival advantage in those receiving intravenous busulfan due to both reduced toxicity and lower rates of relapse[13,14]. Improved outcomes may be achieved when exclusively using intravenous busulfan, and possibly also if ASCT is performed in transplant centers utilizing pharmacokinetic-based busulfan dosing.
In summary, we report here that a Bu/Cy chemotherapy regimen prior to ASCT for relapsed or refractory HL is comparable in toxicity to other published HL ASCT regimens, and demonstrates evidence of anti-lymphoma activity. We suggest that Bu/Cy may be added to the available choices of conditioning chemotherapy prior to ASCT for HL, and may be of particular use in the elderly or in those patients with comorbidities that pose prohibitive risk with other regimens. Prospective, randomized comparison studies are needed to define the optimal regimen(s) that maximizes anti-lymphoma effects while minimizing treatment-related toxicity.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, Chopra R, Milligan D, Hudson GV. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–4. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, Boissevain F, Zschaber R, Muller P, Kirchner H. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065–71. doi: 10.1016/S0140-6736(02)08938-9. others. [DOI] [PubMed] [Google Scholar]
- 4.Kim JG, Sohn SK, Chae YS, Yang DH, Lee JJ, Kim HJ, Shin HJ, Jung JS, Kim WS, Kim DH. Multicenter study of intravenous busulfan, cyclophosphamide, and etoposide (i.v. Bu/Cy/E) as conditioning regimen for autologous stem cell transplantation in patients with non-Hodgkin's lymphoma. Bone Marrow Transplant. 2007;40:919–24. doi: 10.1038/sj.bmt.1705841. others. [DOI] [PubMed] [Google Scholar]
- 5.Wadehra N, Farag S, Bolwell B, Elder P, Penza S, Kalaycio M, Avalos B, Pohlman B, Marcucci G, Sobecks R. Long-term outcome of Hodgkin disease patients following high-dose busulfan, etoposide, cyclophosphamide, and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:1343–9. doi: 10.1016/j.bbmt.2006.08.039. others. [DOI] [PubMed] [Google Scholar]
- 6.Santos EC, Sessions J, Hutcherson D, Flowers C, Langston A, Waller EK. Long-term outcome of Hodgkin disease patients following high-dose busulfan, etoposide, cyclophosphamide, and autologous stem cell transplantation--a similar experience. Biol Blood Marrow Transplant. 2007;13:746–7. doi: 10.1016/j.bbmt.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Ulrickson M, Aldridge J, Kim HT, Hochberg EP, Hammerman P, Dube C, Attar E, Ballen KK, Dey BR, McAfee SL. Busulfan and cyclophosphamide (Bu/Cy) as a preparative regimen for autologous stem cell transplantation in patients with non-Hodgkin lymphoma: a single-institution experience. Biol Blood Marrow Transplant. 2009;15:1447–54. doi: 10.1016/j.bbmt.2009.07.014. others. [DOI] [PubMed] [Google Scholar]
- 8.Talamo G, Claxton DF, Dougherty DW, Ehmann CW, Sivik J, Drabick JJ, Rybka W. BU and CY as conditioning regimen for autologous transplant in patients with multiple myeloma. Bone Marrow Transplant. 2009;44:157–61. doi: 10.1038/bmt.2008.446. [DOI] [PubMed] [Google Scholar]
- 9.Sureda A. Autologous and allogeneic stem cell transplantation in Hodgkin's lymphoma. Hematol Oncol Clin North Am. 2007;21:943–60. doi: 10.1016/j.hoc.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Kuruvilla J, Keating A, Crump M. How I treat relapsed and refractory Hodgkin lymphoma. Blood. doi: 10.1182/blood-2010-09-288373. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf RZ, Dey B, Yeap BY, McAfee S, Attar E, Sepe PS, Dube C, Spitzer TR, Ballen KK. Autologous SCT with a dose-reduced BU and CY regimen in older patients with non-Hodgkin's lymphoma. Bone Marrow Transplant. 2009;43:37–42. doi: 10.1038/bmt.2008.298. [DOI] [PubMed] [Google Scholar]
- 12.Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:523–36. doi: 10.1016/j.bbmt.2008.12.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggarwal C, Gupta S, Vaughan WP, Saylors GB, Salzman DE, Katz RO, Nance AG, Tilden AB, Carabasi MH. Improved outcomes in intermediate- and high-risk aggressive non-Hodgkin lymphoma after autologous hematopoietic stem cell transplantation substituting intravenous for oral busulfan in a busulfan, cyclophosphamide, and etoposide preparative regimen. Biol Blood Marrow Transplant. 2006;12:770–7. doi: 10.1016/j.bbmt.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Dean RM, Pohlman B, Sweetenham JW, Sobecks RM, Kalaycio ME, Smith SD, Copelan EA, Andresen S, Rybicki LA, Curtis J. Superior survival after replacing oral with intravenous busulfan in autologous stem cell transplantation for non-Hodgkin lymphoma with busulfan, cyclophosphamide and etoposide. Br J Haematol. 2010;148:226–34. doi: 10.1111/j.1365-2141.2009.07940.x. others. [DOI] [PubMed] [Google Scholar]