Scheme 2.
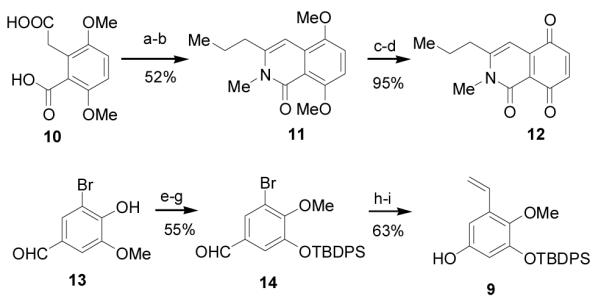
Reagents and conditions: a) 2:1 pyridine: butyric-anhydride reflux; b) 2.0M MeNH2THF RT, CSA, toluene, 85 °C; c) BBr3, THF −78 °C-rt; d) Ag2O, CHCl3; e) AlCl3, pyridine, CH2Cl2 ; f) Li2CO3, MeI, DMF 45 °C; g) TBDPS-Cl, DMAP, TEA, CH2Cl2; h) MCPBA CH2Cl2, NaHCO3, MeOH 0 °C; i) vinyl tributyltin, Pd(PPh3)4, toluene 85 °C. ; THF = tetrahydrofuran, CSA = camphor sulfonic acid, DMAP = 4-dimethylaminopyridine, MCPBA = m-chloroperoxybenzoic acid.
