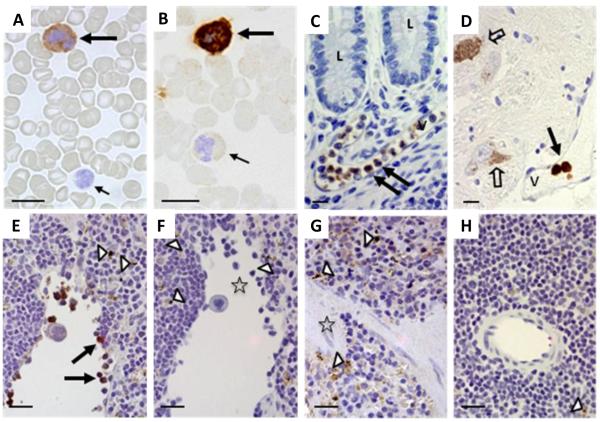
Figure 1. Lrrk2 reactivity in mammalian leukocytes using immunocytochemistry and immunohistochemistry.
(A-D) Lrrk2 reactivity was detected in select leukocytes of peripheral blood smears (A-B; healthy controls; n=5), and in intravascular leukocytes of the submucosa in the distal ileum (C; neurological controls; n=3) and human midbrain (D) from a Parkinson disease donor, as denoted by large arrows. Small arrows point to unstained and poorly stained leukocytes; open arrows identify autofluorescent neuromelanin in dopaminergic cells of the midbrain. V, indicates vessel; L, lumen of the gut. Bar length equals 12 μm; original magnification, 60x. Lrrk2 signal was detected by affinity-purified, polyclonal anti-Lrrk2 antibody [“HL-2” raised against a synthetic peptide comprising residues 2508-2527 of the human, wild-type Lrrk2 (Berger et al. 2010)].
(E-H) The specificity of the Lrrk2 antibody used in A-D was confirmed in spleen sections from age-matched, wild-type (E-F) and lrrk2 knock-out (G-H) mice (n=4 each). Parallel sections were developed with anti-Lrrk2 HL-2 as primary antibody (E; G) or without primary antibody (F; H). Arrows depict lrrk2-immunoreactive leukocytes in the spleen; open arrow heads show autofluorescent cytoplasmic granules seen irrespective of antibody use; open asterisks denote groups of unstained leukocytes (E-H). Bar length equals 20 μm; original magnification, 40x.