Figure 6. INZ inhibits SIRT1 activity and directly binds to SIRT1 in vitro.
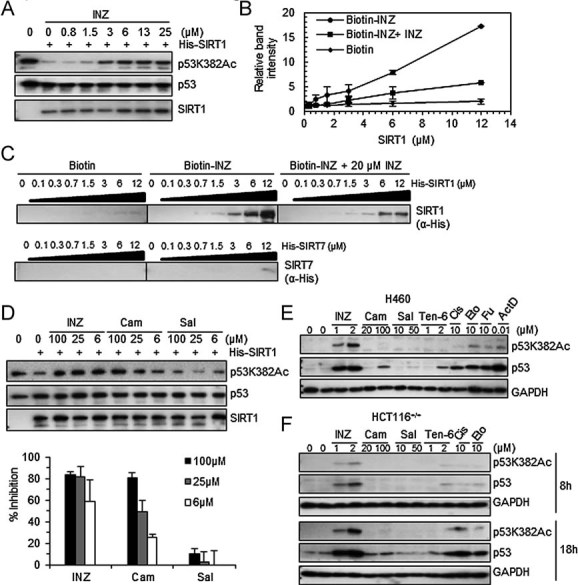
- A. INZ inhibits deacetylation of p53 at lysine 382 by SIRT1 in vitro in a dose-dependent fashion using acetylated p53 protein as a substrate as described in the Experimental Procedures Section of the Supporting Information.
- B-C. Purified SIRT1 was incubated at indicated concentrations with biotinylated INZ that was conjugated with avidin beads in the presence or the absence of 20 µM of non-biotinylated INZ. Purified SIRT7 was used as a negative control. The intensity of each band for bound SIRT1 as analysed using IB was quantified (B), and each sample was individually compared with the intensity of the samples without SIRT1. The results shown are representative of three-independent experiments. Values represent mean ± SD (n = 3).
- D. The inhibitory effects of INZ, Cambinol and Salermide on SIRT1 activity were measured by the increase of the levels of acetylated p53 at lysine 382 in vitro. The percentage of inhibition was calculated as described in the ‘Experimental Procedures’ Section of Supporting Information and shown in below. Values represent mean ± SD (n = 3).
- E-F. Effect of INZ, Cambinol, Salermide or Tenovin-6 on p53 acetylation and level in H460 (E) and HCT116+/+ (F) cells.
