Table 1.
Nitrile Oxides Substituent Dependence in the Production Rearrangement Productsa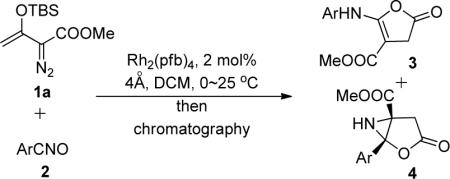
| entry | Ar (2) | product | 3:4b | yield (%)c |
|---|---|---|---|---|
| 1 | 2-MeOC6H4 (2a) | 3a | >95:5 | 92 |
| 2 | 4-MeC6H4 (2b) | 3b, 4b | 90:10 | 84 |
| 3 | 4-FC6H4 (2c) | 3c, 4c | 78:22 | 89 |
| 4 | 4-ClC6H4 (2d) | 3d, 4d | 65:35 | 93 |
| 5 | 4-BrC6H4 (2e) | 3e, 4e | 55:45 | 89 |
| 6 | 4-AcOC6H4 (2f) | 3f, 4f | 39:61 | 81 |
| 7 | 2-NO2C6H4 (2g) | 3g, 4g | <5:95 | 90 |
Reaction conditions: 1a (1.2 mmol) in DCM (3.0 mL) was added over 1 h by syringe pump to mixture of 4 Å molecular sieves (100 mg), Rh2(pfb)4 (2.0 mol %) and nitrile oxide 2 (1.0 mmol) in DCM (5.0 mL) at 0 °C, and stirred for 2 h at room temperature. After solvent removal the product mixture was purified by chromatography on silica gel.
Determined by 1H NMR of the reaction mixture.
Isolated yield of combined 3 and 4 based on limiting reagent 2.
