Table 2.
Rhodium(II) Catalyzed Reaction of Silyl-protected Enoldiazoacetates with Nitnle Oxidesa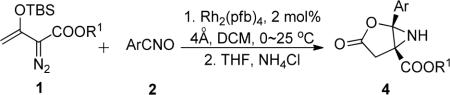
| entry | R1 (1) | Ar (2) | product | yield (%)b |
|---|---|---|---|---|
| 1 | Me (1a) | 2-NO2C6H4 (2g) | 4g | 90 |
| 2 | t-Bu (1b) | 2-NO2C6H4 (2g) | 4h | 92 |
| 3 | Me (1a) | 2,4-(NO2)2C6H4 (2h) | 4i | 81 |
| 4 | t-Bu (1b) | 2,4-(NO2)2C6H4 (2h) | 4j | 88 |
| 5 | Me (1a) | 2-CF3C6H4 (2i) | 4k | 77 |
| 6 | t-Bu (1b) | 2-CF3C6H4 (2i) | 4l | 82 |
Reaction conditions: 1 (1.2 mmol) in DCM (3.0 mL) was added over 1 h via a syringe pump at 0 °C to mixture of 4 Å molecular sieves (100 mg), Rh2(pfb)4 (2.0 mol %) and nitrile oxide 2 (1.0 mmol) in DCM (5.0 mL), and stirred for another 2 h at room temperature. The crude product was hydrolyzed in THF (4.0 mL) with aqueous saturated ammonium chloride solution (2.0 mL) at 60 °C for 1~2 h.
Isolated yield of 4 based on limiting reagent 2.
