Abstract
Arylalkylamine N-acetyltransferase (AANAT) is the key regulatory enzyme controlling the daily rhythm of melatonin biosynthesis. In chicken retinal photoreceptor cells, Aanat transcription and AANAT activity are regulated in part by cAMP-dependent mechanisms. The purpose of this study was to identify regulatory elements within the chicken Aanat promoter responsible for cAMP-dependent induction. Photoreceptor-enriched retinal cell cultures were transfected with a luciferase reporter construct containing up to 4kb of 5′-flanking region and the first exon of Aanat. Forskolin treatment stimulated luciferase activity driven by the ~4kb promoter construct and by all 5′-deletion constructs except the smallest, Aanat (−217 to +120)luc. Maximal basal and forskolin-stimulated expression levels were generated by the Aanat (−484 to +120)luc construct. This construct lacks a canonical cyclic AMP-response element (CRE), but contains two other potentially important elements in its sequence: an eight times TTATT repeat (TTATT8) and a CRE-like sequence (CLS). Electrophoretic mobility shift assays (EMSA), luciferase reporter assays, chromatin immunoprecipitation, and siRNA experiments provide evidence that these elements bind c-Fos, JunD, and CREB to enhance basal and forskolin-stimulated Aanat transcription. We propose that the CLS and TTATT8 elements in the 484 bp proximal promoter interact to mediate cAMP-dependent transcriptional regulation of Aanat.
Keywords: CRE, AP1, cAMP, CREB, circadian rhythm, arylalkylamine N-acetyltransferase, Aanat, gene regulation, retina, melatonin, JunD, cFos
Arylalkylamine N-acetyltransferase (AANAT) regulates the daily rhythm in the biosynthesis of melatonin in the pineal gland and retina (Klein 2007; Klein et al. 1997). In chicken retina, AANAT is expressed primarily in photoreceptor cells (Bernard et al. 1997). cAMP stimulates AANAT activity in chicken retina and in cultured chicken photoreceptor cells through transcriptional and post-translational mechanisms (Iuvone et al. 1990; Zawilska et al. 1992; Greve et al. 1999; Pozdeyev et al. 2006).
The best characterized mechanism of cAMP-dependent gene expression in vertebrates involves the cAMP response element binding protein (CREB), a 43-kDa basic leucine zipper transcription factor, which binds to the cAMP-response element (CRE), an 8-bp palindromic cis-acting element (5′-TGACGTCA-3′) located in the regulatory regions of target genes (Meyer and Habener 1993; Montminy 1997; Mayr and Montminy 2001). Genes responding to cAMP usually contain one or more CRE sequences in their promoters. Although CREB can bind to CREs it promotes transcription only when phosphorylated by cAMP-dependent protein kinase (PKA) (Yamamoto et al. 1988; Gonzalez and Montminy 1989) and other Ser/Thr protein kinases (Du and Montminy 1998). There are several alternatively spliced isoforms of CREB of which CREB341 (CREB1) and CREB327 (Δ-CREB, lacking 14 amino acids) are expressed in most tissues (Brindle and Montminy 1992). CREB binds to the CRE either as a homodimer or as a heterodimer in combination with other members of the CREB/ATF and AP-1 families of transcription factors (Montminy and Bilezikjian 1987; Hoeffler et al. 1988).
A previous study demonstrated that cAMP increases the abundance of the Aanat transcript in cultured chick retinal photoreceptor cells and that this effect can be blocked by actinomycin D (Greve et al. 1999). Studies on the rat Aanat gene have revealed that a CRE-like sequence (CLS; TGCGCCA)-CCAAT complex in the 5′-flanking region and a canonical CRE in the first intron drive cAMP-dependent induction (Baler et al. 1997; Baler et al. 1999; Burke et al. 1999). The CLS has alternatively been referred to as natCRE and CRE2, and is 7/8 identical to the canonical CRE. In contrast, the chicken Aanat gene does not contain a canonical CRE sequence TGACGTCA (Chong et al. 2000) raising the possibility that a CLS in the chicken Aanat promoter mediates cAMP-dependent expression of Aanat. Alternatively, an indirect mechanism involving transcription factors other than or in addition to CREB may contribute to expression.
In the present study, we addressed the question of how cAMP regulates Aanat expression in the chicken retina by characterizing its 5′-flanking region. We provide evidence for functional roles of a novel TTATT repeat sequence and a CLS (6/8 identical to the canonical CRE) in basal and cAMP-driven promoter activity. We also provide evidence that CREB, c-Fos, and Jun-D are components of protein complexes that interact with these sequences.
Materials and Methods
Cell cultures
Photoreceptor-enriched retinal cell cultures were prepared from embryonic day 6 to 6.5 chicks (Gallus domesticus) and cultured as described by Adler et al. (1984) with minor modifications (Iuvone et al. 1990; Avendano et al. 1990).
Transfection of retinal cells and luciferase reporter assay
Primary retinal cell cultures were transfected with the pGL3 luciferase reporter vector (Promega, Madison, WI) containing Aanat promoter constructs. Retinal cells were transfected with one of the following constructs (−3999 to +120, −1633 to +120, −1309 to +120, −484 to +120 and −217 to +120; Gene Bank accession no. AF193072), prepared as described previously (Chong et al. 2000). The vector pGL3 basic was used as a background control and the pRL-TK Renilla luciferase reporter was used as an internal control. On the day of transfection (5th day in culture), medium was replaced with 4 ml of fresh medium containing 5% fetal bovine serum and antibiotics. DNA used for transfection was diluted with transfection buffer and mixed with Effectene™ (Qiagen Inc., Valencia, CA) according to the manufacturer’s protocol. The transfection complex mixed with 1 ml culture medium was added to the dishes. Cells were incubated at 37°C and 5% CO2 in air for 24 or 48 hours (hrs). Forskolin (10 μM) was added for the last 6 hrs to stimulate cAMP levels. Cell lysates were subsequently assayed for firefly and Renilla luciferase activities using the Dual-Luciferase Reporter Assay (Promega, Madison, WI) and a luminometer (Turner Designs TD20/20, Sunnyvale, CA). The firefly luciferase activity was normalized to constitutively expressed Renilla luciferase activity in each sample, and fold induction was calculated with respectto activity in cells that were transfected with an empty vector.
Site-directed mutagenesis of the Aanat promoter
The GeneEditor in vitro site-directed mutagenesis system from Promega (Madison, WI) was used to generate mutations in TTATT region and CLS sequence. The vector pGL3-Aanat was alkaline denatured for 20 min at RT followed by annealing to the mutagenic oligonucleotides and the bottom strand selection oligonucleotide provided by the kit supplier. The sequences of the primers used for deletion mutation are presented in Table 1. The remaining procedure was performed as described in the GeneEditor protocol (Promega). All constructs were confirmed by Sanger sequencing (SeqWright, Houston, TX).
Table 1.
Oligonucleotides and primers used for Aanat promoter constructs, EMSA, site-directed mutagenesis, qRT-PCR, and ChIP PCR
| Name | Sequence | |
|---|---|---|
| Position (bp) | ||
| Oligonucleotides for EMSA assay: | ||
| TTATT8 | 5′-TTATTTTATTTTATTTTATTTTATTTTATTTTATTTTATT-3′ | |
| −468/−429 | ||
| TTATT7 | 5′-TTATTTTATTTTATTTTATTTTATTTTATTTTATT-3′ | |
| −468/−434 | ||
| TTATT6 | 5′-TTATTTTATTTTATTTTATTTTATTTTATT-3′ | |
| −468/−439 | ||
| TTATT5 | 5′-TTATTTTATTTTATTTTATTTTATT-3′ | |
| −468/−444 | ||
| TTATT4 | 5′-TTATTTTATTTTATTTTATT-3′ | |
| −468/−449 | ||
| CLS | 5′-AGGCAGTCACGCCAGGGTAAAG-3′ | |
| −176/−155 | ||
| CRE-Con | 5′-AGGCAGTGACGTCAGGGTAAAG -3′ | |
| −176/−155 | ||
| mCLS | 5′-AGGCAGTCacgcCAGGGTAAAG-3′ | |
| −176/−155 | ||
| Primers to generate two AANAT promoter constructs: | ||
| TTATT0 (−428+120bp)-sense | 5′-AATCATTACTTCTGCTGACCTTCCCG -3′ | |
| −425/−400 | ||
| TTATT1 (−433+120bp)-sense | 5′-TTATTTTTAATCATTACTTCTGCTGACCTTC -3′ | |
| −433/−403 | ||
| TTATT0/TTATT1-antisense | 5′-CGGCTGTGGGATGGGGACCT-3 | |
| +110/+120 | ||
| Primers for site-directed mutagenesis: | ||
| mTTATT5 | 5′-CAGAAGGCTTATTTTATTTTATTTTATTTTATTttattttattttatt | |
| TTTAAT | −476/−397 | |
| CATTACTTCTGCTGACCTTCCCGGTT-3′ | ||
| mTTATT4 | 5′-CAGAAGGC TTATTTTATTTTATTTTATT | |
| ttattttattttattttattTTTAAT | −476/−400 | |
| CATTACTTCTGCTGACCTTCCCG -3′ | ||
| mCLS | 5′-TTTATCCCCCCTCAGGCAGTCacgcCAGGGTAAAGAAA- | |
| TCAGCCTCAGC-3′ | −189/−141 | |
| Primers for qRT-PCR: | ||
| c-Fos forward: | 5′-CATCTGTGAGAGCTGGTAGTCT-3′ | |
| c-Fos reverse: | 5′-CCAGCACCAGGTTAATTCCAATCA-3′ | |
| Aanat forward: | 5′-CTGATGTGGCGGTACCTGCAGT-3′ | |
| Aanat reverse: | 5′-ACCTCGTGCTGCATCTCGGTG-3′ | |
| Creb1 forward: | 5′-CCTGGACCTGCTTCTTGTGTTACA-3′ | |
| Creb1 reverse: | 5′-CCTACAGTAATGACACGACGGACTA-3′ | |
| Hprt forward: | 5′-GGACAGAGAGACTGGCACGT-3′ | |
| 167 | ||
| Hprt reverse: | 5′-ATGAAGTCCACAGTCATGGGG-3′ | |
| Primers for ChIP PCR: | ||
| TTATT-sense | 5′-TGCCTTGAGTTGCTCTGAAATGCC-3′ | |
| −511/−488 | ||
| TTATT-antisense | 5′-TCCCGAGAGTGAGCTCCTGG-3′ | |
| −387/−367 | ||
| CLS - sense | 5′-CCAGCACGCAGCAATCAGCTAAA-3′ | |
| −217/−194 | ||
| CLS - antisense | 5′-CGGAGCCTCGCTTATATGTCTGTT-3′ | |
| −33/−10 | ||
Underlined are the CRE and CLS sequences; lower case letters indicate deleted bases.
DNA-protein binding assay
Preparation of nuclear extract
Unless noted otherwise, photoreceptor cultures were treated with 10 μM forskolin for 6 hrs prior to collecting cells. Nuclear proteins were prepared from the cells using the Nuclear Extract Kit (Active Motif, Carlsbad, CA) according to manufacturer’s instructions. Nuclear extracts were stored at −80°C until used.
Electrophoretic mobility shift assay (EMSA)
The polyacrylamide gel electrophoresis (PAGE)-purified sense and antisense oligonucleotides (Integrated DNA Technologies, Inc., Coralville, IA) were dissolved in the following buffer: 10 mM Tris pH 8.0, 50 mM NaCl, 1 mM EDTA. The two strands were mixed together in equal molar amounts, heated to 94°C for 5 min and incubated at room temperature (RT) (~22° C) for 30 min for annealing. The double-stranded oligonucleotides were end-labeled with γ-[32P] ATP using T4 polynucleotide kinase (Promega). The sequences of the probes used for TTATT and CLS gel shift assays are presented in Table 1. 32P-labeled double stranded probe (1 ng) was incubated for 30 min at RT in 1x binding buffer (10 mM Tris-HCl pH 7.5, 50 mM NaCl, 1 mM MgCl2, 0.5 mM dithiothreitrol, 0.5 mM EDTA, 5% glycerol, and 1 μg of poly dIdC) containing 30 μg of nuclear extracts. For competition experiments and antibody supershift, the competing unlabeled probes (50-fold molar excess) and antibodies were preincubated for 20 min at RT with the nuclear extracts before the addition of the radiolabeled probes. Following the addition of the probe, the binding mixtures were incubated for 20 min at RT. The protein-DNA complexes were resolved on 6% non-denaturing polyacrylamide gels for 1 to 2 hrs at 250V in 0.05 M Tris, 0.045 M Boric acid, 0.5 mM EDTA-pH 8.4. Before loading the samples, the gel was pre-run for 30 min at 150V. After electrophoresis, the gel was fixed, dried and exposed to autoradiography film at −80°C for 5 hrs to overnight.
Antibodies directed against the following antigens were used in EMSA analyses: mouse JAB1 and Fra-2 (cat. # sc-135954 and sc-604; Santa Cruz Biotechnolog, Santa Cruz, CA); human JunD (cat. # ab-425; Abcam, Cambridge, MA); pCREB (cat. # 9190S; Cell Signaling Technology, Inc., Danvers, MA), human Δ-CREB (Williams et al. 1993; Dr. Ourania Andrisani, Purdue University, West Lafayette, Indiana); and chicken c-Fos (Bussolino et al. 1998, Dr. Beatriz Caputto, Universidad Nacional de Cordoba, Argentina).
Chromatin Immunoprecipitation (ChIP)
Formaldehyde cross-linking and ChIP were performed as described previously (Haque et al. 2010). Immunoprecipitations were performed with Protein G-agarose and 2 μg of the indicated antibodies: anti-phosphoCREB (cat.# 9190S; Cell Signaling Technology, Beverly, MA); anti-Δ-CREB (Williams et al. 1993); anti-c-Fos (Bussolino et al. 1998), and anti-JunD (cat. # ab425; Abcam, Cambridge, MA). Negative controls included no antibody and normal rabbit IgG instead of the specific antibody. For ChIP qPCR, 3 μl of ChIP DNA and Input DNA (diluted 1:10) were used. The sequences of the primers used for ChIP PCR are presented in Table 1. The amplification conditions were the following: 5 min at 95°C, 40 cycles at 95°C for 10 s and 60°C for 30 s. Each sample was assayed in duplicate, and the fluorescence threshold value (Ct) was calculated using MyiQ cycler software (Bio-Rad Laboratories, Hercules, CA). The qPCR analysis of ChIP DNA was normalized with that of normal IgG.
Creb and c-Fos siRNA transfection
Primary chicken retinal cells were transfected with Creb and c-Fos siRNAs using Lipofectamine™ 2000 according to the manufacturer’s protocol (Invitrogen Corp., Carlsbad, CA). The knockdown of endogenous Creb and c-Fos was performed using the pre-designed siRNA from Santa Cruz (sc-270088 and sc-270089; Santa Cruz Biotechnology Inc., Santa Cruz, CA). c-Fos and Creb siRNAs are a pool of 3 target-specific 19–25 siRNAs designed to knock down gene expression. The siRNA sequences used to target the chicken c-Fos were sense: 5′-CUAGUGCAGCUGAUUAACAtt-3′, antisense: 5′-UGUUAAUCAGCUGCACUAGtt-3′; sense: 5′-GUCUGAAUGUUCUGACAUAtt-3′, antisense: 5′-UAUGUCAGAACAUUCAGACtt-3′; sense: 5′-CCAUUAAGUGCAGAUUUGUtt-3′, antisense: 5′-ACAAAUCUGCACUUAAUGGtt-3′; and the siRNA sequences used to target the chicken Creb were sense: 5′-GCAAUUCAGUUGUCUAACAtt-3′, antisense: 5′-UGUUAGACAACUGAAUUGCtt-3′; sense: 5′-CAACCAAGUUGUAGUACAAtt-3′, antisense: 5′-UUGUACUACAACUUGGUUGtt-3′; sense: 5′-CAACGGUGUUGUUUAGUAAtt-3′, antisense: 5′-UUACUAAACAACACCGUUGtt-3′. LacZ siRNA was used as negative control. Creb and c-Fos siRNAs at a final concentration of 50 nM were co-transfected with luciferase reporter plasmids into chicken retinal cells. Transfectants were maintained in the culture medium for 24 hrs before being used in qRT-PCR analysis and in the luciferase activity assay. The protocols for transfection, forskolin stimulation of the cells, and assays for firefly and Renilla luciferase activities are described above. The firefly luciferase activity of each of the chicken 484-Aanat promoter construct was determined by calculating luciferase activity relative to the activity of the Renilla luciferase, and fold induction was calculated with respect to the activity of cells transfected with empty vector.
Quantitative RT-PCR (qRT-PCR)
c-Fos, Creb1, and Aanat transcripts were quantified by qRT-PCR using 2 μl of cDNA from each sample. qRT-PCR was performed in MyiQ Cycler (Bio-Rad Laboratories Inc., Hercules, CA) with a 25 μl total volume containing cDNA, 1x QuantiFast SYBR Green PCR Master mix (Qiagen), and 300 nM gene-specific forward and reverse primers. The primer sequences used in qPCR analyses of each gene are presented in Table 1. The amplification conditions were the following: 5 min at 95°C, 40 cycles at 95°C for 20 s and 60°C for 30 s. Each sample was assayed in duplicate, and the fluorescence threshold value (Ct) was calculated using MyiQ cycler software. The levels of c-Fos, Creb1, and Aanat transcripts were normalized to the expression levels of the housekeeping gene Hprt and quantified according to the delta-delta Ct method (Livat and Schmittgen 2001).
Data analysis
Statistical analysis among groups was performed using one-way or two-way analysis of variance (ANOVA) with Student-Newman-Keuls multiple comparison test where applicable. Data are expressed as the mean ± SEM.
Results
Promoter analysis of Aanat gene
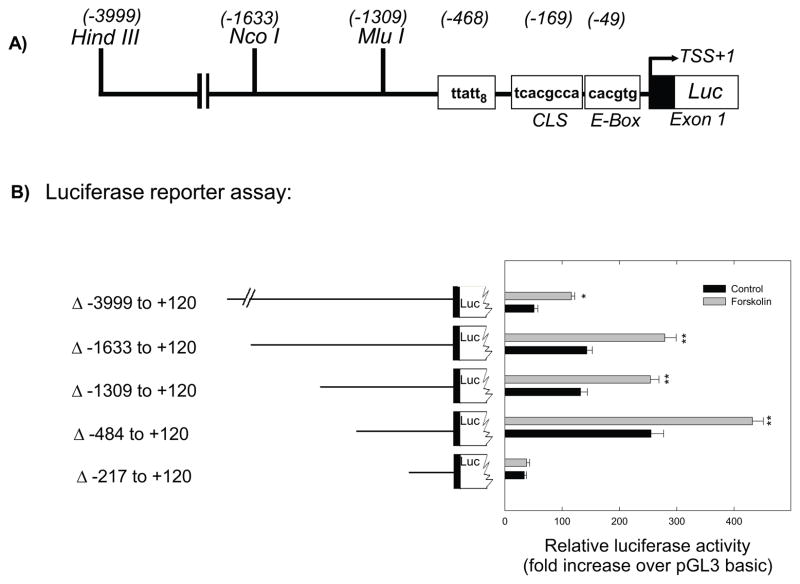
Luciferase reporter assays were used to examine regions of the Aanat promoter responsible for basal and forskolin-induced gene expression (Fig. 1). Treatment with forskolin (10 μM) significantly increased luciferase activity driven by the ~4 Kb promoter construct, Aanat (−3999 to +120)luc, and by all 5′-deletion constructs except the smallest, Aanat (−217 to +120)luc (Fig. 1). Both basal and forskolin-stimulated expression levels were maximal with Aanat (−484 to +120)luc (Fig. 1). Analysis of Aanat (−484 to +120) reveals that it contains three potentially important cis-acting elements (Fig. 2): an eight times repeat of TTATT (TTATT8), a CRE-like sequence (CLS; 5′-TCACGCCA-3′), and a circadian E-box (CACGTG) that mediates circadian expression driven by the clock proteins BMAL1, CLOCK, and NPAS2 (Chong et al. 2000; Haque et al. 2010). The basal expression in retinal cells of Aanat (−217 to +120)luc, which contains the CLS and the E-box, but not the TTATT8 sequence, was minimal and completely unresponsive to forskolin (Fig. 1), indicating that a sequence upstream of the CLS is essential for basal and cAMP stimulated transcription.
Fig. 1.
Functional analysis of the chicken Aanat promoter. A. Schematic representation of a partial restriction map of the 5′-flanking region of the Aanat gene showing unique restriction sites. Potential regulatory elements are boxed between position −1309 and −1, relative to the transcription start site (TSS). B. Luciferase reporter assay. Fragments of the Aanat promoter were ligated to the firefly luciferase gene (pGL3-basic) and transfected into primary retinal cells. Each well was transfected with 1.0 μg luciferase construct DNA and 0.1 μg of control DNA (pRL-TK-Renilla-luciferase vector, Promega) using Effectene (Qiagen) transfection reagent. Numbers represent position relative to the TSS. Cells were incubated for 48 h with forskolin (10 μM) added during the last 6 hours. Data represent the mean ± SEM fold increase in luciferase activity relative to control (pGL3-basic) untreated cells; n=4 per condition. Two-way ANOVA indicated a significant effects of treatment (vehicle vs forskolin; p<0.001), construct length (p<0.001), and a significant interaction of treatment and length (p<0.001); *p=0.002 vs vehicle; **p<0.001 vs vehicle. For further details see Materials and Methods.
Fig. 2.
The 484 bp proximal promoter region of the Aanat (GenBank accession number AF193072) and putative transcription factor binding sites. The sequences of double-stranded oligonucleotides used for EMSA experiments are shown underlined. Bases are numbered such that +1 represents the start of first exonic sequence. The octameric CRE-like binding site (CLS), 5′-TCACGCCA-3′, is in bold font. The consensus E-box sequence is in italics.
Analysis of the TTATT motif
EMSAs were done to characterize interactions of a double-stranded oligonucleotide corresponding to the TTATT8 sequence, nucleotides (nt) −468 to −428 (Fig. 2), with nuclear proteins from forskolin-treated cells. The radiolabeled TTATT8 probe recruited proteins that separated into three complexes (I, II, and III) when resolved on polyacrylamide gels for 1 hr (Fig. 3). Competition studies using unlabeled consensus oligonucleotides as competitors confirmed binding specificity. In order to determine the number of TTATT repeats required for protein complexes to bind with the probes, we made five separate probes containing 4–8 repeats: TTATT8, TTATT7, TTATT6, TTATT5, and TTATT4 (Fig. 3). Strong binding of proteins to the probes was observed with TTATT8, TTATT7, and TTATT6 oligonucleotides, though the binding of protein complex I was markedly reduced with TTATT6. The TTATT5 probe did not generate protein complex I, and protein complexes II and III bound to this oligo were significantly reduced. The TTATT4 probe did not recruit any of the protein complexes at all, which suggests that at least five TTATT repeats in the Aanat proximal promoter are required for proteins to bind with the oligos. The protein complexes generated from various TTATT probes were effectively competed in the presence of a 50-fold molar excess of TTATT8 unlabeled oligonucleotides.
Fig. 3.
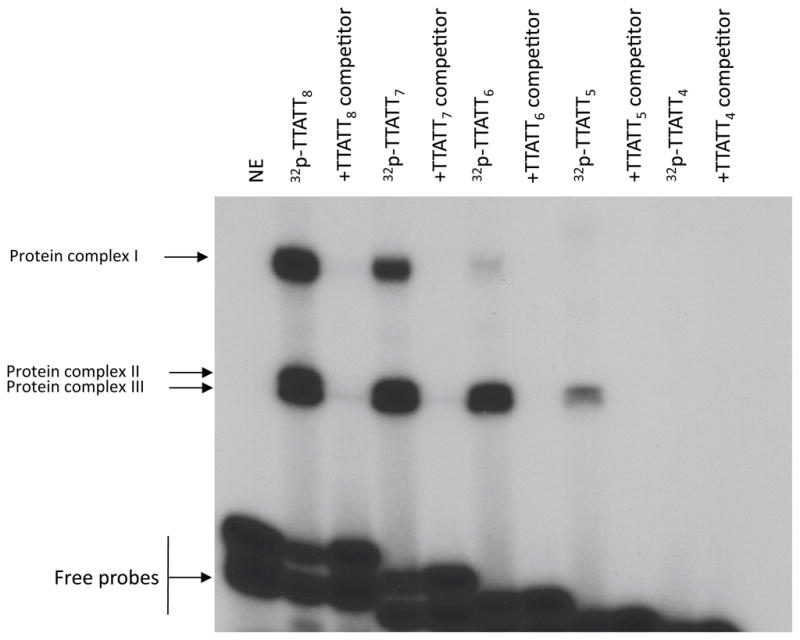
Gel mobility shift assay of nuclear proteins from retinal cells binding to TTATT repeats of AANAT proximal promoter. Sequences of various lengths of TTATT repeats used as probes or competitors. Gel shift assay was carried out by incubating 30 μg nuclear extract from retinal cells with probes TTATT8, TTATT7, TTATT6, TTATT5, and TTATT4. Sequences of various lengths of TTATT repeats used as probes are presented in Table 1. Competition assay was performed in the presence of a 50-fold molar excess of unlabeled TTATT8 oligonucleotide, as indicated on top. The binding reactions were resolved by electrophoresis on a 6% polyacrylamide gel for 1 hour. The arrows indicate the DNA/protein complexes (protein complexes I–III) and the free probes. Nuclear protein was not added to reactions labeled-NE. For further details see Materials and Methods.
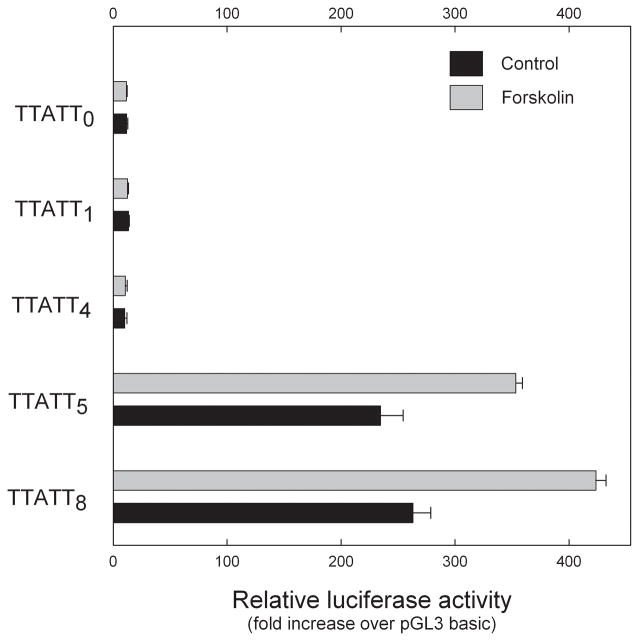
A requirement for at least five repeats of TTATT for promoter activity was indicated by luciferase reporter assays (Fig. 4). Deletion of four of the eight TTATT repeats from the −484 to +120 reporter construct drastically reduced basal promoter activity and eliminated forskolin stimulation. The promoter construct with five times repeat of TTATT elicited basal luciferase activity that was not significantly different from the activities of the construct containing eight repeats of TTATT (Fig. 4). However, forskolin-stimulated luciferase activity of the TTATT5 construct was significantly lower than that of the promoter construct containing all eight of the TTATT repeats (P<0.01).
Fig. 4.
Five or more TTATT repeats are required for basal and forskoiln-stimulated Aanat luciferase reporter activity. Chicken retinal cells were transiently transfected with 1μg of pGL3 reporter plasmids containing zero, one, four, five and eight blocks of TTATTand the remainder of the Aanat .....-+120 lucI reporter sequence. After 48 h, luciferase activities were determined as described in Materials and Methods. Cells were stimulated with forskolin at a final concentration of 10 μM for 6 hours. Each value shown represents the mean ± SEM for five independent transfection experiments. Levels of luciferase expression were normalized to Renilla luciferase activity of cotransfected 0.1 μg pRL-TK plasmid. Two-way ANOVA indicated a significant effects of treatment (vehicle vs forskolin; p<0.001), construct length (p<0.001), and a significant interaction of treatment and length (p<0.001); p<0.001 vs vehicle. For further details see Materials and Methods.
Analysis of CLS element
To determine whether nuclear proteins interact with the CLS site, nuclear extracts from retinal cells were incubated with a radiolabeled double-stranded DNA oligonucleotide corresponding to the Aanat CLS sequence with Aanat flanking sequences, nucleotides (nt) −176 to −154 (Fig. 2). The radiolabeled CLS probe recruited two protein complexes (A and B) from nuclear extracts of forskolin-treated cells (Fig. 5). A probe with the consensus CRE plus Aanat flanking sequences also produced two protein complexes with the same mobility as CLS (Fig. 5), although the binding was less intense compared to the CLS probe. Competition experiments revealed that both protein complexes were displaced by a 50-fold molar excess of unlabeled CLS oligonucleotide (Fig. 5), consistent with direct binding of the protein complexes to the CLS probe.
Fig. 5.
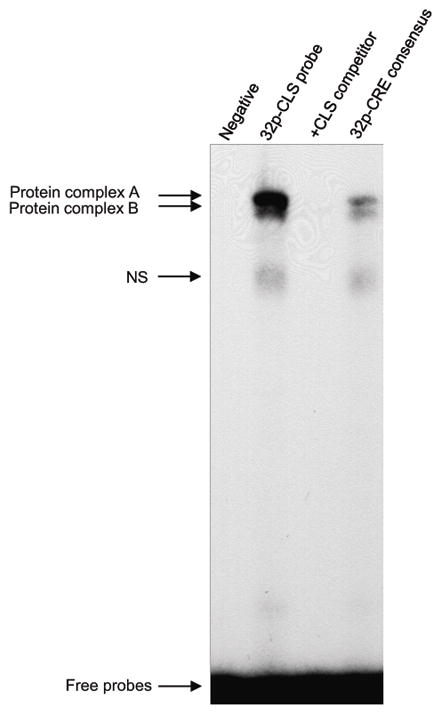
EMSA analysis of proteins interacting with the Aanat CLS and a CRE consensus sequence. Retinal cell nuclear protein extract (30 μg) was incubated with a 32P-labeled CLS probe in the absence (none) or presence of a 50-fold molar excess of unlabeled CLS competitor, or with a 32P-labeled consensus CRE with Aanat flanking sequences. The probe and competitor sequences are presented in Table 1. DNA-protein complexes were separated by 6% non-denaturing PAGE electrophoresis for 1 hr and were visualized by autoradiography. The arrows indicate DNA-protein complexes (A, B, and a non-specific [NS] band) and the free probes. Nuclear protein was not added to reactions labeled ‘Negative’. For further details see Materials and Methods.
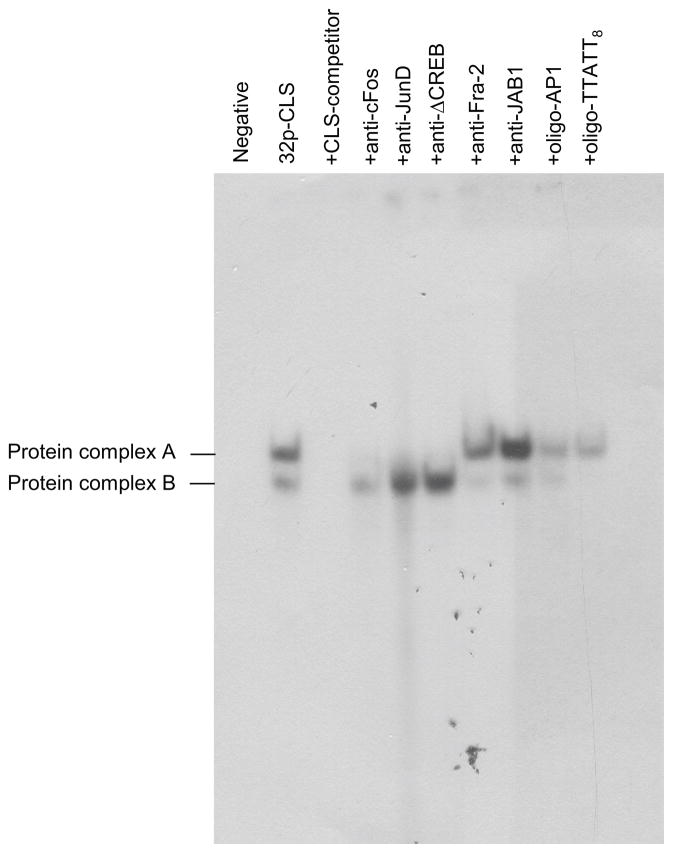
The unlabeled TTATT8 oligonucleotide also completely displaced protein complex B (Fig. 6), suggesting a possible interaction between these sites. The binding of protein complex A to the CLS oligonucleotide was inhibited by antisera directed against c-Fos, JunD, and Δ-CREB (Fig. 6). Other antibodies tested and an AP1 oligonucleotide competitor appeared to have relatively minor effects on protein complex formation.
Fig. 6.
CREB, c-Fos, and JunD antibodies and a TTATT8 oligonucleotide bind with CLS-bound protein complexes: Competition assays with EMSA were performed by incubating retinal nuclear extract with a radiolabeled CLS probe. Competition analysis was performed in the presence of 50-fold excess of unlabeled oligonucleotides CLS, AP1, and TTATT8. Two CLS specific DNA protein complexes (see Fig. 5) are indicated as A and B. To identify specific proteins, five antibodies, anti-c-Fos, anti-JunD, anti-ΔCREB, anti-Fra-2, and anti-JAB1 were used in this EMSA assay. None of the antibodies produced a supershift in the protein complexes, but anti-c-Fos, anti-JunD, and anti-ΔCREB completely inhibited binding of protein complex A. AP1 oligonucleotide reduced binding of nuclear proteins to CLS, and the competitor TTATT8 oligonucleotide completely displaced protein complex B. The gel was run for 2 hours. For further details see the Materials and Methods.
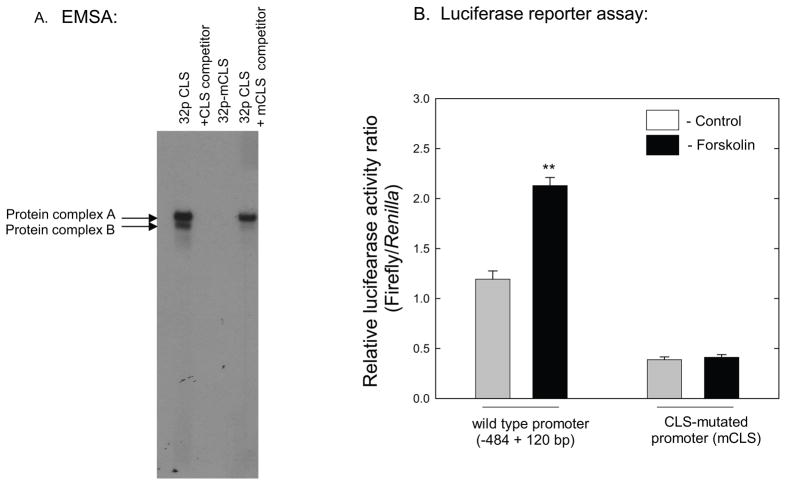
To determine if the CLS sequence is required for induction by cAMP, a mutant form of CLS (mCLS) was used in EMSA and luciferase reporter assays. Gel-shift experiments were performed with a CLS oligonucleotide in which four nucleotides from the core of the CLS sequence were deleted (Table 1). No protein complexes were observed using the mCLS probe (Fig. 7A). In addition, an unlabeled mutated CLS oligonucleotide with four nucleotides deleted from CRE-like core sequence failed to compete with binding of proteins to the wild-type CLS probe at a 50-fold molar excess (Fig. 7A). Deletion of these four nucleotides from the core of the CLS in the Aanat (−484 to +120)luc reporter construct decreased basal promoter activity (~70%) and eliminated the response to forskolin (Fig. 7B).
Fig. 7.
Mutation of CLS sequence and Aanat promoter activity. A. EMSA was performed by incubating retinal nuclear extract with radiolabeled CLS wild type and mutant (mCLS) probes. The sequences of CLS and mCLS are presented in Table 1. Competition analysis was performed in the presence of 50-fold excess of unlabeled CLS wild type and mutant oligonucleotides. B. Chicken retinal cells were transiently transfected with 1μg of the wild type Aanat (−484+120) luciferase construct or one containing mCLS. After 48 h, luciferase activities were determined. Cells were stimulated by forskolin (10 μM) for the last 6 hours. Levels of firefly luciferase expression were normalized to Renilla luciferase activity of the co-transfected pRL-TK plasmid. Data represent the mean ± SEM fold-induction in luciferase activity relative to control (pGL3-basic) cells. **p<0.01 vs. all other groups; n=4 per condition. For further details see Materials and Methods.
Chromatin Immunoprecipitation (ChIP) of the Aanat promoter
To test whether CREB and AP1 transcription factors directly bind to the TTATT8 region and CLS sequence of the Aanat promoterin retinal cells, ChIP assays were done using specific antibodies against CREB, c-Fos, and JunD to immunoprecipitate formaldehyde-fixed chromatin from forskolin-treated retinal cells. As shown in Fig. 8, the DNA from cross-linked chromatin/transcription factor immuno-precipitates was detected by qPCR amplification with oligonucleotides spanning bases −511 to −367 and −217 to −10 of the chicken Aanat promoter that encompass TTATT8 region and CLS sequence, respectively, indicating that CREB, c-Fos, and JunD directly interact with the proximal promoter in retinal cells.
Fig. 8.
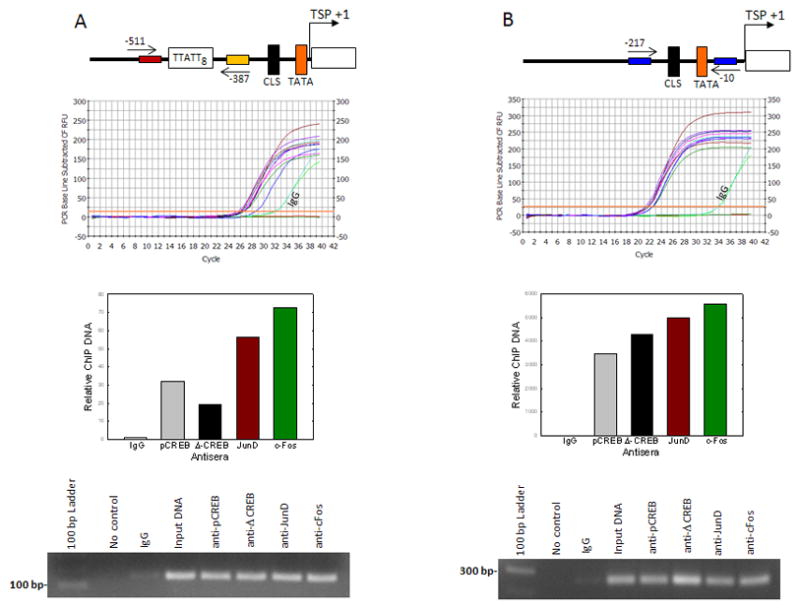
In situ association of CREB, c-Fos, and JunD proteins with the Aanat promoter region containing TTATT8 and CLS cis-elements. Retinal cells were treated for 6 hrs with forskolin (10 μM) and then incubated with 1% formaldehyde to cross-link endogenous proteins and DNA. Upper panels: Illustration of Aanat promoter region amplified for ChIP assay. Arrows mark the location of primers used to amplify Aanat promoter region. The relative positions of TTATT8, CLS, and TATA in the Aanat promoter region is shown (A-B). Middle panels: Cellular DNA fragments were immunoprecipitated with anti-pCREB, anti-Δ–CREB, anti-JunD, anti-c-Fos, or normal IgG, and DNA fragments spanning −511 to −367 bp (A) and −217 to −10 (B), encompassing the TTATT8 and CLS respectively, were amplified by qPCR. Primer pairs against Aanat promoter were used in Input control (diluted 1:10), and the primers without ChIP DNA were used as a ‘no DNA control’ (A–B). The histograms represent relative quantity of DNA immunoprecipitated by specific antibodies normalized to that recovered with normal IgG, as measured by ΔΔCt (lower-middle panels); IgG values were set to 1. Lower panels: PCR products were run in 1% agarose gel (A–B). For further details see Materials and Methods.
CREB and c-Fos activate Aanat transcription in retinal cells
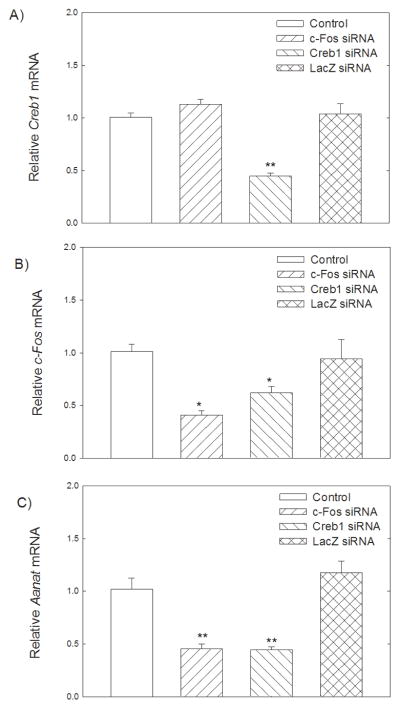
To further investigate the regulatoryinfluence of c-Fos and Creb on Aanat, we silenced c-Fos and Creb geneexpression in forskolin-treated retinal cells. Retinal cells were transfected with Creb and c-Fos siRNAs at final concentrations of 50 nM. The knockdown of Creb and c-Fos was confirmed by real-time PCR. Creb and c-Fos siRNAs significantly downregulated the Creb (p<0.001) and c-Fos (p=0.005) mRNA levels (Fig. 9A, B). In addition, Creb siRNA significantly reduced c-Fos mRNA (p<0.05) suggesting that CREB regulates c-Fos expression (Fig. 9B). The effect of the c-Fos and Creb silencing on Aanat gene expression was analyzed by real-time PCR. We observed that the silencing of either c-Fos or Creb resulted in a significant decrease in Aanat gene expression compared with the controls (p<0.001), indicative of the involvement of c-Fos and Creb in the regulation of Aanat expression (Fig. 9C). To extend our study of the roles of c-Fos and CREB, retinal cells were co-transfected with Aanat (-484+120)luc and c-Fos/Creb siRNAs (50 nM). As shown in Fig. 10, addition of forskolin (10 μM) to the cells significantly increased luciferase activity of Aanat (−484+120)luc (p<0.001). Co-transfection with Creb or c-Fos siRNAs significantly reduced basal Aanat (−484+120)luc activity (p<0.001) and eliminated stimulation by forskolin (p<0.001).
Fig. 9.
RNA interference-mediated suppression of Creb and c-Fos expression on Aanat, Creb and c-Fos mRNA levels. Retinal cells were transfected with 50 ng of Creb siRNA, c-Fos siRNA, or lacZ siRNA, and incubated for 24 hours before harvesting for total RNA extraction; forskolin (10μM) was added for the last 6 h. Creb, c-Fos, and Aanat levels were expressed relative to Hprt mRNA with the value of the mock-transfected control set to 1. Values are presented as mean ± SEM; n=5. A. Transfection of siRNA complementary to Creb mRNA led to a significant decrease of Creb (**p<0.001 vs. control). B. Transfection of siRNAs complementary to c-Fos mRNA or Creb mRNA significantly decreased c-Fos transcript level (#p=0.005, *p<0.05). C. Transfection of siRNAs complementary to c-Fos mRNA or Creb mRNA significantly decreased Aanat transcript level (**p<0.001). For further details see Materials and Methods.
Fig. 10.
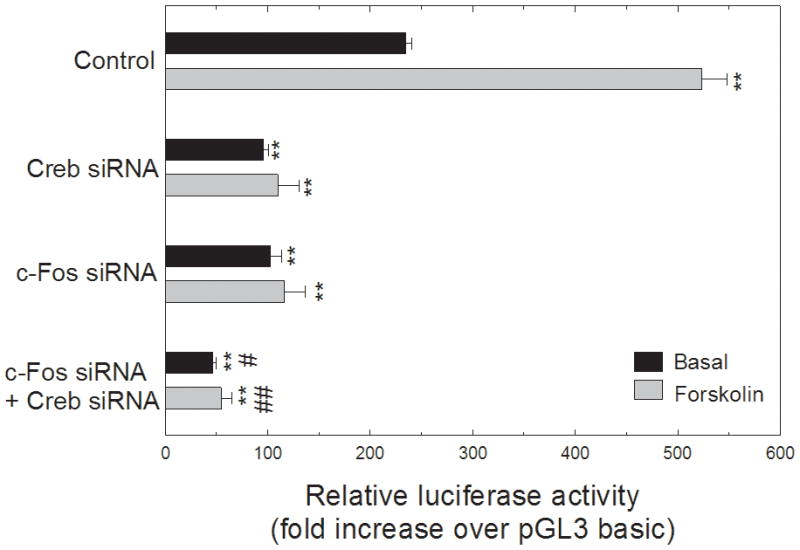
Knockdown of Creb and c-Fos reduced luciferase activity of Aanat (−484+120)luc reporter-transfected retinal cells. Cells were transfected with 50 ng of Creb and c-Fos siRNAs. For each well of cells, 1.0 μg of the Aanat (−484+120)luc construct was co-transfected with 0.1 μg of pRL-TK vector that provides constitutive expression of Renilla luciferase serving as an internal control. Cells with vectors and siRNA’s were incubated for 24 hours, and were stimulated by forskolin at a final concentration of 10 μM for 6 hours before harvesting. Luciferase activities were determined by dual luciferase reporter assay system. Levels of firefly luciferase expression were normalized to Renilla luciferase activity of co-transfected pRL-TK plasmid. The luciferase activity of the Aanat promoter construct is expressed as fold change over pGL3-basic vector. Values are presented as mean ± SEM, n=3–4. Two-way ANOVA indicated a significant effects of treatment (vehicle vs forskolin; p<0.001), siRNA (p<0.001), and a significant interaction of treatment and siRNA (p<0.001);, **p<0.001 vs. control; #p<0.05 vs. Creb siRNA or c-Fos siRNA; ##p<0.05 vs. Creb siRNA + forskolin or c-Fos siRNA + forskolin. For further details see Materials and Methods.
Discussion
The results of this investigation indicate that the regulatory region of the Aanat promoter that confers the strongest basal and forskolin-stimulated expression in photoreceptors is located in the first 484 nucleotides of the 5′-flanking region of the gene. This minimal promoter contains a CLS, a TTATT8 motif, and a circadian E-box enhancer element. Our studies indicate that the CLS and TTATT8 elements support both basal and forskolin-stimulated promoter activity. We have also found that the TTATT8 region and CLS bind protein complexes, and that the composition of these complexes points to a role of AP1 and CREB transcription factors in the regulation of Aanat transcription via the minimal promoter.
The TTATT8 motif represents a novel nuclear protein binding sequence. Not all eight TTATT repeats are essential for nuclear protein binding or for promoter activity. However, deletion of more than three TTATT repeats eliminates nuclear protein binding, reduces basal promoter activity by ~90%, and completely blocks forskolin-stimulated transcription. c-Fos, JunD, and CREB have been identified as candidate transcription factors binding to this novel motif. The TTATT8 motif was not found in Aanat genes from other species, including mouse, rat, and zebrafish. A similar, though not identical T/A-rich region is present in the human Aanat gene, 374 to 352 nucleotides upstream of the transcriptional start site (Coon et al. 1996; NCBI GenBank U40391). BLAST searches (http://blast.ncbi.nlm.nih.gov/Blast.cgi) revealed many examples of 4–8 TTATT repeats within the human, chicken, and mouse genome databases, but it is unknown if these represent functional promoter elements. Although the TTATT8 motif appears to be a novel promoter element in the chicken Aanat gene, we cannot exclude the possibility that it contains other promoter elements within the sequence and/or 3′-flanking region that could be affected by deletion of the four TTATT repeats.
The sequence of CLS in the Aanat promoter, 5′-TCACGCCA-3′, differs from the canonical CRE sequence, 5′-TGACGTCA-3′ (Roesler et al. 1988; Roesler, 2000), by two nucleotides. We have shown that this sequence binds nuclear proteins from photoreceptor cultures. Deletion of four nucleotides from the core of CLS eliminated nuclear protein binding to the sequence. This deletion also reduced basal activity of the Aanat (−484+120)luc reporter construct by ~70% and eliminated forskolin stimulation. This mutated reporter contains the intact TTATT8 motif; thus, both CLS and TTATT8 appear to be required for strong basal and cAMP-stimulated promoter activity. Binding of nuclear proteins to CLS was greater than that to a canonical CRE with identical flanking sequences, suggesting specificity in binding to this sequence. The present results provide multiple lines of evidence that CREB, c-Fos, and JunD bind to CLS.
CREB-1 and Δ-CREB bind to non-canonical CREs with one to three nucleotide substitutions, usually with lower affinity and transactivation potential (Williams et al. 1993; Roesler et al. 1988; Benbrook and Jones 1994). Gel shift and ChIP assays indicated that Δ-CREB binds to the CLS of the Aanat promoter. Δ-CREB has 10-fold lower activity than CREB-1 in some promoters (Yamamoto et al. 1990). This may explain the relatively weak stimulation of chicken Aanat promoter activity by cAMP compared to that of the rat Aanat gene, which contains a canonical CRE in the first intron and a near perfect (7 of 8 nucleotides) CRE-CCAAT complex in the proximal promoter (Baler et al. 1997; Baler et al. 1999), collectively accounting for tissue specific expression in the pineal gland and retina and strong cAMP induction (Burke et al. 1999). Intronic E-box sequences also contribute to tissue specificity of rat Aanat (Humphries et al., 2007).
Our experiments with Creb and c-Fos siRNAs confirm the involvement of CREB and c-Fos transcription factors in the regulation of Aanat expression. siRNA directed against either transcription factor reduced Aanat expression. Interestingly, the Creb siRNA reduced the levels not only of Creb and Aanat transcripts, but also of c-Fos mRNA, indicating that CREB regulates c-Fos in photoreceptor cells. This observation is consistent with previous studies in other cell types showing that the cAMP-CREB signaling system regulates c-Fos gene expression via binding of pCREB to the promoter (Herdegen and Leah 1998; Hao et al. 2008; Coulon et al. 2010).
Several lines of evidence converge to suggest that TTATT8 and CLS interact to regulate basal and cAMP-stimulated Aanat promoter activity. Neither site alone is capable of sustaining promoter activity. Binding of nuclear proteins to CLS was displaced by competition with unlabelled TTATT8 oligonucleotide. Anti-cFos inhibits binding of Complex A to CLS. Complex A binding is also disrupted by anti-JunD, suggesting that it may partner with cFos to regulate these sites.
The expression of Aanat mRNA in chicken retina exhibits a circadian rhythm with highest levels at night (Bernard et al. 1997). This rhythm is thought to be generated by cycling clock proteins acting on the E-box enhancer element in the proximal promoter (Chong et al. 2000; Haque et al. 2010). However, the CLS may also contribute to this circadian regulation. There are circadian rhythms of cAMP level and CREB phosphorylation in photoreceptors with high levels at night (Liu and Green 2002; Ivanova and Iuvone 2003). c-Fos mRNA and protein are under circadian control in photoreceptors, also with high levels at night (Bussolino et al. 1998; Yoshida et al. 1993; Humphries and Carter 2004). JunD, but not cJun or JunB, is constitutively expressed in photoreceptors (Imaki et al. 1995). A circadian clock in retina generates a daily rhythm of the type 1 adenylyl cyclase and thereby gates photoreceptor melatonin biosynthesis (Fukuhara et al. 2004; Chaurasia et al. 2006). Thus, a complex of c-Fos/JunD/pCREB may augment E-box activated transcription of Aanat at night via actions on the CLS and TTATT8 regulatory sites. Additional experiments are needed to test this hypothesis and its relevance to in vivo control of retinal melatonin biosynthesis.
In summary, we have identified novel promoter elements in the proximal promoter that regulate basal and cAMP-stimulated transcriptional activities of the Aanat gene. These elements appear to bind c-Fos, JunD, and CREB. The CLS element acts in concert with the TTATT8 motif to enhance basal and cAMP-induced transcription activity of the promoter.
Acknowledgments
The research was supported by NIH grants R01EY004864 (PMI), P30EY006360 (PMI), funds from the Intramural Research Program of the Eunice Shriver Kennedy National Institute of Child Health and Human Development (DCK), and an unrestricted departmental grant from Research to Prevent Blindness (RPB). PMI is a recipient of the Senior Scientific Investigator Award from RPB. NWS is supported by the BBSRC UK (G18273). The authors gratefully thank Dr. Beatriz Caputto and Dr. Ourania Andrisani for donating c-Fos and Δ-CREB antisera, respectively. The authors also thank Zhuping Xu, Jane Abey, and Aamra Thazyeen, for providing technical assistance. A preliminary report of a portion of these data was presented in abstract form (Haque et al., 2004).
Footnotes
The authors declare no conflict of interest with respect to the research reported herein.
References
- Adler R, Lindsey JD, Elsner CL. Expression of cone-like properties by chick embryo neural retina cells in glial-free monolayer cultures. J Cell Biol. 1984;99:1173–1178. doi: 10.1083/jcb.99.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avendano G, Butler BJ, Iuvone PM. K(+)-evoked depolarization induces serotonin N- acetyltransferase activity in photoreceptor-enriched retinal cell cultures. Involvement of calcium influx through l-type channels. Neurochem Int. 1990;17:117–126. doi: 10.1016/0197-0186(90)90075-5. [DOI] [PubMed] [Google Scholar]
- Baler R, Covington S, Klein DC. The rat arylalkylamine N-acetyltransferase gene promoter. cAMP activation via a cAMP-responsive element-CCAAT complex. J Biol Chem. 1997;272:6979–6985. doi: 10.1074/jbc.272.11.6979. [DOI] [PubMed] [Google Scholar]
- Baler R, Covington S, Klein DC. Rat arylalkylamine N-acetyltransferase gene: upstream and intronic components of a bipartite promoter. Biol Cell. 1999;91:699–705. [PubMed] [Google Scholar]
- Benbrook DM, Jones NC. Different binding specificities and transactivation of variant CRE’s by CREB complexes. Nucleic Acids Res. 1994;22:1463–1469. doi: 10.1093/nar/22.8.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard M, Iuvone PM, Cassone VM, Roseboom PH, Coon SL, Klein DC. Avian melatonin synthesis: photic and circadian regulation of serotonin N-acetyltransferase mRNA in the chicken pineal gland and retina. J Neurochem. 1997;68:213–224. doi: 10.1046/j.1471-4159.1997.68010213.x. [DOI] [PubMed] [Google Scholar]
- Brindle PK, Montminy MR. The CREB family of transcription activators. Curr Opin Genet Dev. 1992;2:199–204. doi: 10.1016/s0959-437x(05)80274-6. [DOI] [PubMed] [Google Scholar]
- Burke Z, Wells T, Carter D, Klein D, Baler R. Genetic targeting: the serotonin N-acetyltransferase promoter imparts circadian expression selectively in the pineal gland and retina of transgenic rats. J Neurochem. 1999;73:1343–1349. doi: 10.1046/j.1471-4159.1999.0731343.x. [DOI] [PubMed] [Google Scholar]
- Bussolino DF, de Arriba Zerpa GA, Grabois VR, Conde CB, Guido ME, Caputto BL. Light affects c-fos expression and phospholipid synthesis in both retinal ganglion cells and photoreceptor cells in an opposite way for each cell type. Brain Res Mol Brain Res. 1998;58:10–15. doi: 10.1016/s0169-328x(98)00065-5. [DOI] [PubMed] [Google Scholar]
- Chaurasia SS, Haque R, Pozdeyev N, Jackson CR, Iuvone PM. Temporal coupling of cyclic AMP and Ca/calmodulin-stimulated adenylyl cyclase to the circadian clock in chick photoreceptor cells. J Neurochem. 2006;99:1142–1150. doi: 10.1111/j.1471-4159.2006.04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong NW, Bernard M, Klein DC. Characterization of the chicken serotonin N-acetyltransferase gene. Activation via clock gene heterodimer/E box interaction. J Biol Chem. 2000;275:32991–32998. doi: 10.1074/jbc.M005671200. [DOI] [PubMed] [Google Scholar]
- Coon SL, Mazuruk K, Bernard M, Roseboom PH, Klein DC, Rodriguez IR. The human serotonin N-acetyltransferase (EC 2.3.1.87) gene (AANAT): structure, chromosomal localization, and tissue expression. Genomics. 1996;34:76–84. doi: 10.1006/geno.1996.0243. [DOI] [PubMed] [Google Scholar]
- Coulon V, Chebli K, Cavelier P, Blanchard JM. A novel mouse c-fos intronic promoter that responds to CREB and AP-1 is developmentally regulated in vivo. PLoS One. 2010;5:e11235. doi: 10.1371/journal.pone.0011235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Liu C, Ivanova TN, Chan GCK, Storm DR, Iuvone PM, Tosini G. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J Neurosci. 2004;24:1803–1811. doi: 10.1523/JNEUROSCI.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier BR, Schwitzgebel VM, Zaiko M, Mamin A, Ritz-Laser B, Philippe J. Hepatic nuclear factor-3 (HNF-3 or Foxa2) regulates glucagon gene transcription by binding to the G1 and G2 promoter elements. Mol Endocrinol. 2002;16:170–83. doi: 10.1210/mend.16.1.0752. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Greve P, Alonso-Gomez A, Bernard M, Ma M, Haque R, Klein DC, Iuvone PM. Serotonin N- acetyltransferase mRNA levels in photoreceptor-enriched chicken retinal cell cultures: elevation by cyclic AMP. J Neurochem. 1999;73:1894–1900. [PubMed] [Google Scholar]
- Hao H, Liu H, Gonye G, Schwaber JS. A fast carrier chromatin immunoprecipitation method applicable to microdissected tissue samples. J Neurosci Methods. 2008;172:38–42. doi: 10.1016/j.jneumeth.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Chong NW, Chaurasia SS, Klein DC, Iuvone PM. Retinal melatonin biosynthesis: Interactions of A/T–rich regions and CRE–like sequences contribute to cAMP–dependent regulation of the chicken AANAT promoter. Invest Ophthalmol Vis Sci. 2004;45:E-Abstract 659. [Google Scholar]
- Haque R, Ali FG, Biscoglia R, Abey J, Weller J, Klein D, Iuvone PM. CLOCK and NPAS2 have overlapping roles in the circadian oscillation of arylalkylamine N-acetyltransferase (Aanat) mRNA in chicken cone photoreceptors. J Neurochem. 2010;113:1296–1306. doi: 10.1111/j.1471-4159.2010.06698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hoeffler JP, Meyer TE, Yun Y, Jameson JL, Habener JF. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988;242:1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- Humphries A, Carter DA. Circadian dependency of nocturnal immediate-early protein induction in rat retina. Biochem Biophys Res Commun. 2004;320:551–556. doi: 10.1016/j.bbrc.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Humphries A, Wells T, Baler R, Klein DC, Carter DA. Rodent Aanat: intronic E-box sequences control tissue specificity but not rhythmic expression in the pineal gland. Mol Cell Endocrinol. 2007;270:43–49. doi: 10.1016/j.mce.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Imaki J, Yamashita K, Yamakawa A, Yoshida K. Expression of jun family genes in rat retinal cells: regulation by light/dark cycle. Brain Res Mol Brain Res. 1995;30:48–52. doi: 10.1016/0169-328x(94)00270-o. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Avendano G, Butler BJ, Adler R. Cyclic AMP-dependent induction of serotonin N-acetyltransferase activity in photoreceptor-enriched chick retinal cell cultures: characterization and inhibition by dopamine. J Neurochem. 1990;55:673–682. doi: 10.1111/j.1471-4159.1990.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Iuvone PM. Circadian rhythm and photic control of cAMP level in chick retinal cell cultures: a mechanism for coupling the circadian oscillator to the melatonin- synthesizing enzyme, arylalkylamine N-acetyltransferase, in photoreceptor cells. Brain Res. 2003;991:96–103. doi: 10.1016/j.brainres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriguez IR, Bégay V, Falcón J, Cahill GM, Cassone VM, Baler R. Recent. Prog Horm Res. 1997;52:307–357. [PubMed] [Google Scholar]
- Klein DC. Arylalkylamine N-Acetyltransferase: “the Timezyme”. J Biol Chem. 2007;282:4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- Liu X, Green CB. Circadian regulation of nocturnin transcription by phosphorylated CREB in Xenopus retinal photoreceptor cells. Mol Cell Biol. 2002;22:7501–7511. doi: 10.1128/MCB.22.21.7501-7511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. Review. [DOI] [PubMed] [Google Scholar]
- Meyer TE, Habener JF. Cyclic adenosine 3′,5′-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocr Rev. 1993;14:269–290. doi: 10.1210/edrv-14-3-269. [DOI] [PubMed] [Google Scholar]
- Montminy MR. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- Montminy MR, Bilezikjian LM. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987;328:175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Pozdeyev N, Taylor C, Haque R, Chaurasia SS, Visser A, Thazyeen A, Du Y, Fu H, Weller J, Klein DC, Iuvone PM. Photic regulation of arylalkylamine N-acetyltransferase binding to 14-3-3 proteins in retinal photoreceptor cells. J Neurosci. 2006;26:9153–9161. doi: 10.1523/JNEUROSCI.1384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler WJ, Vandenbark GR, Hanson RW. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988;263:9063–9066. [PubMed] [Google Scholar]
- Roesler WJ. What is a cAMP response unit? Mol Cell Endocrinol. 2000;162:1–7. doi: 10.1016/s0303-7207(00)00198-2. [DOI] [PubMed] [Google Scholar]
- Swanson DJ, Zellmer E, Lewis EJ. AP1 proteins mediate the cAMP response of the dopamine beta-hydroxylase gene. J Biol Chem. 1998;273:24065–74. doi: 10.1074/jbc.273.37.24065. [DOI] [PubMed] [Google Scholar]
- Williams JS, Dixon JE, Andrisani OM. Binding constant determination studies utilizing recombinant delta CREB protein. DNA Cell Biol. 1993;12:183–190. doi: 10.1089/dna.1993.12.183. [DOI] [PubMed] [Google Scholar]
- Yamamoto KK, Gonzalez GA, Biggs WH, 3rd, Montminy MR. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988;334:494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto KK, Gonzalez GA, Menzel P, Rivier J, Montminy MR. Characterization of a bipartite activator domain in transcription factor CREB. Cell. 1990;60:611–617. doi: 10.1016/0092-8674(90)90664-z. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kawamura K, Imaki J. Differential expression of c-fos mRNA in rat retinal cells: regulation by light/dark cycle. Neuron. 1993;10:1049–1054. doi: 10.1016/0896-6273(93)90053-t. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Wawrocka M, Zurawska E, Nowak JZ. Calcium channel blockers in vivo inhibit serotonin N-acetyltransferase (NAT) activity in chicken retina stimulated by darkness and not by agents elevating intracellular cyclic AMP level. J Pineal Res. 1992;13:101–6. doi: 10.1111/j.1600-079x.1992.tb00062.x. [DOI] [PubMed] [Google Scholar]