Figure 7.
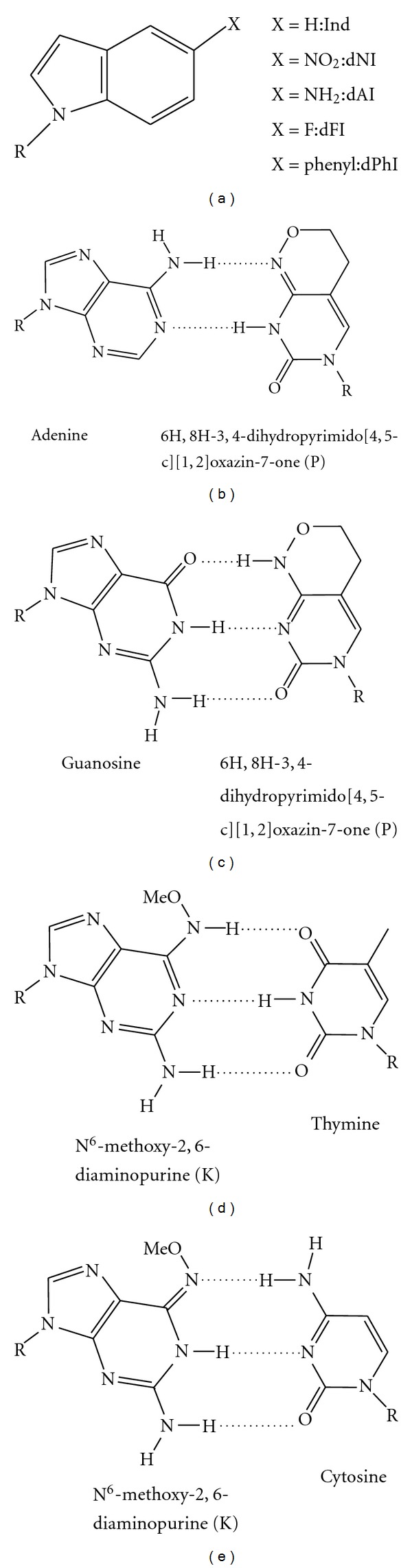
Purine and pyrimidine mimics. (a) Purine analogs based on the indole scaffold: 5-substituted indolyl-2′deoxyriboside triphosphates where X = H, indole (Ind); X = NO2, 5-nitro-1-indole (dNI); X = NH2, 5-amino-1-indole (dAI); X = F, 5-fluoro-1-indole (dFI); X = phenyl, 5-phenyl-1-indole (dPhI) [95, 96]. ((b)–(e)) Pyrimidine mimic 6H,8H-3,4-dihydropyrimido[4,5-c][1,2]oxazin-7-one (P), and purine mimic N 6-methoxy-2,6-diaminopurine (K) base pairing partners. (b) Adenine : P. (c) Guanosine : P. (d) Thymine : K. (e) Cytosine : K. The ability of these analogs to form different tautomers can result in mutations [98].
