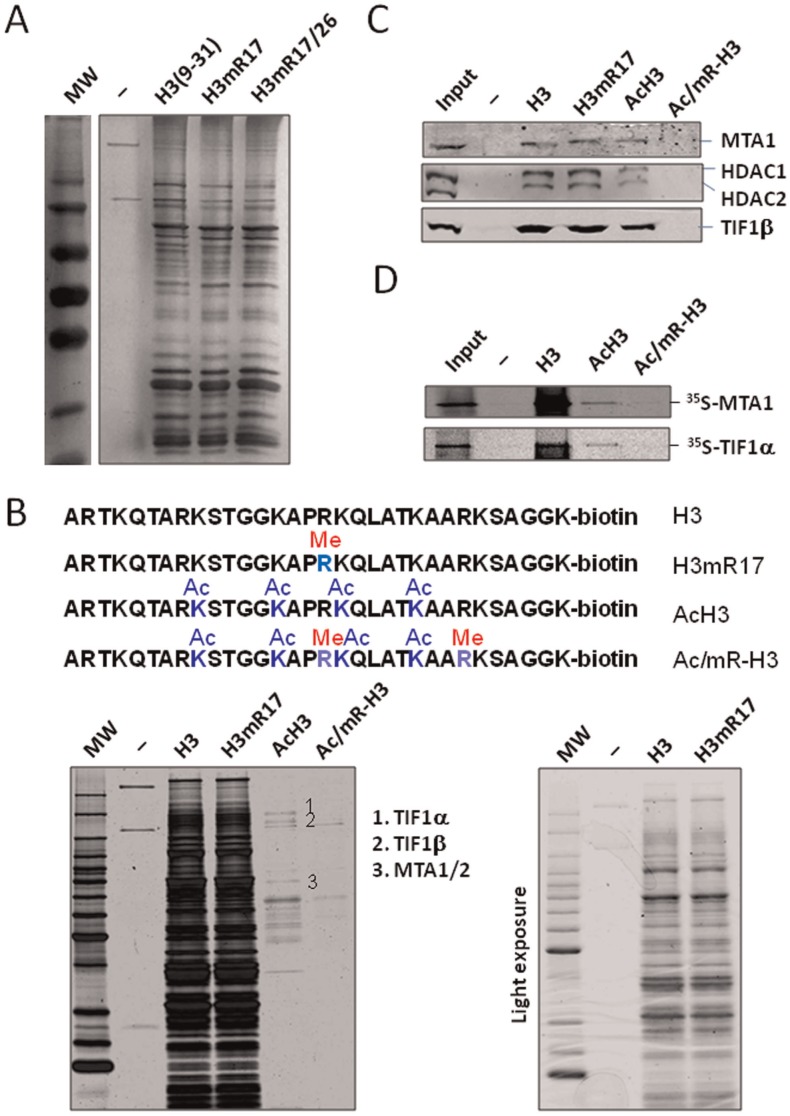
Figure 1. H3R17me2a and H3R26me2a inhibit binding of NuRD and TIF1 family proteins to acetylated H3 tail peptide.
(A) Purification of HeLa nuclear proteins using immobilized control and H3 peptides containing either R17me2a (H3mR17) or R17me2a and R26me2a double methylation (H3mR17/26). All peptides correspond to H3 N-terminal tail amino acids 9–29 and have three additional amino acids GGK plus a C-terminal Biotin. The peptides were first immobilized to streptavidin agarose beads and then incubated with HeLa nuclear extracts. The proteins retained by the control beads (−) or immobilized peptides were resolved by 10% SDS-PAGE and visualized by silver staining. (B) Purification of HeLa nuclear proteins using H3 peptides containing either acetylation (AcH3) or both acetylation and R17/R26me2a (Ac/mR-H3). All peptides correspond to H3 tail amino acids 1–29. The bound proteins were resolved by 10% SDS-PAGE and revealed by silver staining. The major bands that were present in the lane of AcH3 but absent in the lane of Ac/mR-H3 were excised and identified by mass spectrometry analysis. The light exposure was shown for proteins bound to H3 and H3 mR17 peptide. Note R17me2a alone did not appear to affect the binding of numerous proteins to H3 peptide. (C) Western blot analysis confirmed the inhibitory effect of H3R17/26me2a on the binding of NuRD complex and TIF1β to acetylated H3 peptide. The proteins bound to various immobilized peptides were analyzed by western blotting using antibodies as indicated. (D) In vitro synthesized MTA1 and TIF1α bound poorly to Ac/mR-H3. MTA1 and TIF1α were synthesized and radiolabled with 35S-methionine using transcription and translation coupled reaction (Promega) and subjected to pulldown assay using peptides as indicated. The binding of MTA1 and TIF1α was revealed by autoradiography.