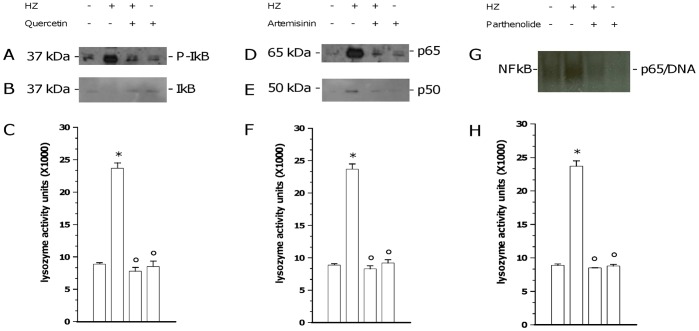
Figure 5. Involvement of NF-kappaB pathway in HZ-dependent induction of lysozyme release from human adherent monocytes.
Cells were unfed (negative controls) or fed with HZ (positive controls) for 2 h; after phagocytosis, cells were incubated for 2 h alone and with: 15 microM quercetin, an inhibitor of cytosolic I-kappaBalpha protein phosphorylation (Panels A–C); 10 microM artemisinin, an inhibitor of p65 and p50 NF-kappaB subunits nuclear translocation (Panels D–F); and 10 microM parthenolide, an inhibitor of DNA/NF-kappaB complex binding (Panels G–H). Therefore, I-kappaBalpha protein phosphorylation (A) and degradation (B), along with p65 (D) and p50 (E) nuclear translocation were evaluated by WB in cytosolic and nuclear fractions of cell lysates, respectively; DNA/NF-kappaB complex binding (G) was evaluated by EMSA in nuclear fraction of cell lysates; and lysozyme release was measured by spectrometric assay in cell supernatants (C, F, H). Results are shown as representative images (WB and EMSA studies) or means + SEM (lysozyme release studies) of three independent experiments. Lysozyme release from monocytes is indicated as enzyme activity units measured in a 2 ml-well of cell supernatants. In lysozyme release studies, data were also evaluated for significance by ANOVA. Panel C: Vs unstimulated cells (column 1) *p<0.0001; Vs untreated HZ-fed cells (column 2) °p<0.0001. Panel F: Vs unstimulated cells (column 1) *p<0.0001; Vs untreated HZ-fed cells (column 2) °p<0.0001. Panel H: Vs unstimulated cells (column 1) *p<0.0001; Vs untreated HZ-fed cells (column 2) °p<0.0001.