TABLE I.
Current or planned trials involving targeted agents relevant to patients with non-small-cell lung cancer
| Targeted agent | ClinicalTrials.gov ID | Phase | Status | Population | Estimated enrollment (n) | Estimated completion date | Trial site |
|---|---|---|---|---|---|---|---|
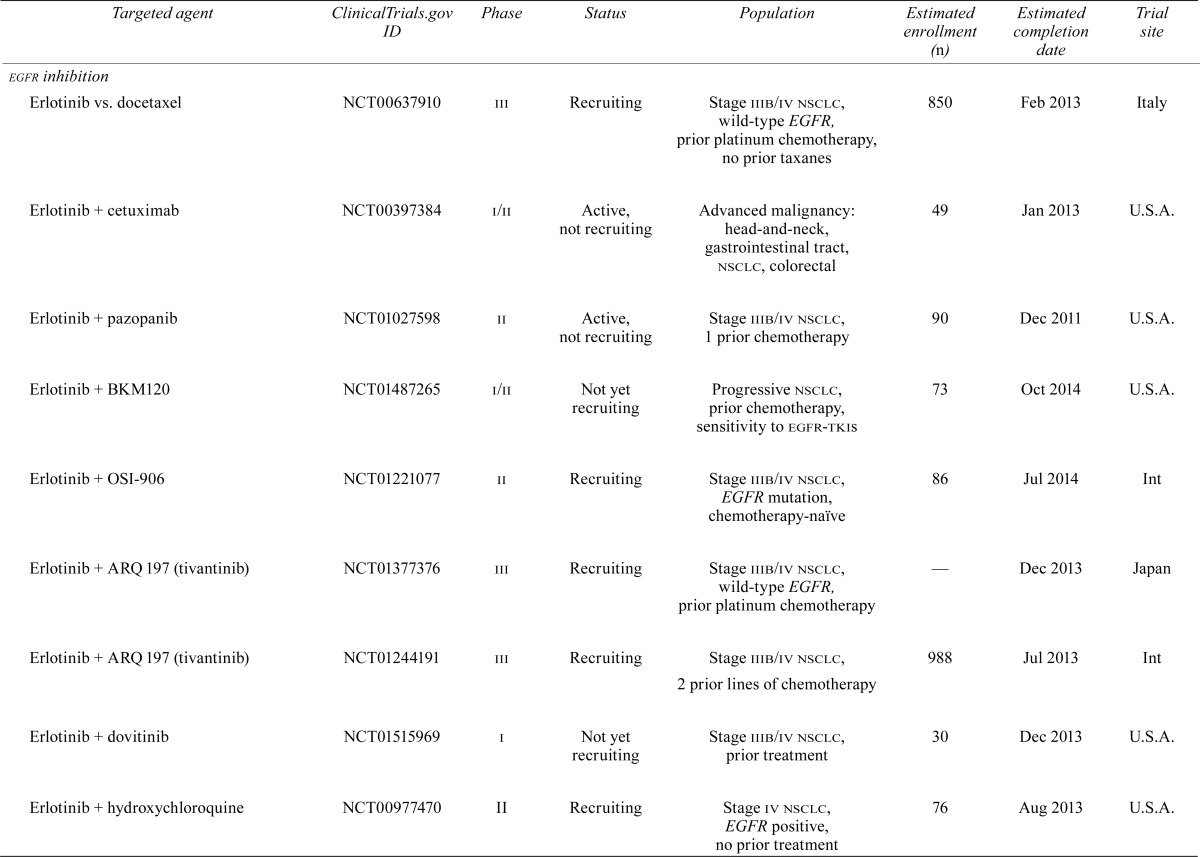
| egfr inhibition | |||||||
| Erlotinib vs. docetaxel | NCT00637910 | iii | Recruiting | Stage iiib/iv nsclc, wild-type EGFR, prior platinum chemotherapy, no prior taxanes | 850 | Feb 2013 | Italy |
| Erlotinib + cetuximab | NCT00397384 | i/ii | Active, not recruiting | Advanced malignancy: head-and-neck, gastrointestinal tract, nsclc, colorectal | 49 | Jan 2013 | U.S.A. |
| Erlotinib + pazopanib | NCT01027598 | ii | Active, not recruiting | Stage iiib/iv nsclc, 1 prior chemotherapy | 90 | Dec 2011 | U.S.A. |
| Erlotinib + BKM120 | NCT01487265 | i/ii | Not yet recruiting | Progressive nsclc, prior chemotherapy, sensitivity to egfr-tkis | 73 | Oct 2014 | U.S.A. |
| Erlotinib + OSI-906 | NCT01221077 | ii | Recruiting | Stage iiib/iv nsclc, EGFR mutation, chemotherapy-naïve | 86 | Jul 2014 | Int |
| Erlotinib + ARQ 197 (tivantinib) | NCT01377376 | iii | Recruiting | Stage iiib/iv nsclc, wild-type EGFR, prior platinum chemotherapy | — | Dec 2013 | Japan |
| Erlotinib + ARQ 197 (tivantinib) | NCT01244191 | iii | Recruiting | Stage iiib/iv nsclc, 2 prior lines of chemotherapy | 988 | Jul 2 013 | Int |
| Erlotinib + dovitinib | NCT01515969 | i | Not yet recruiting | Stage iiib/iv nsclc, prior treatment | 30 | Dec 2013 | U.S.A. |
| Erlotinib + hydroxychloroquine | NCT00977470 | ii | Recruiting | Stage iv nsclc, EGFR positive, no prior treatment | 76 | Aug 2013 | U.S.A. |
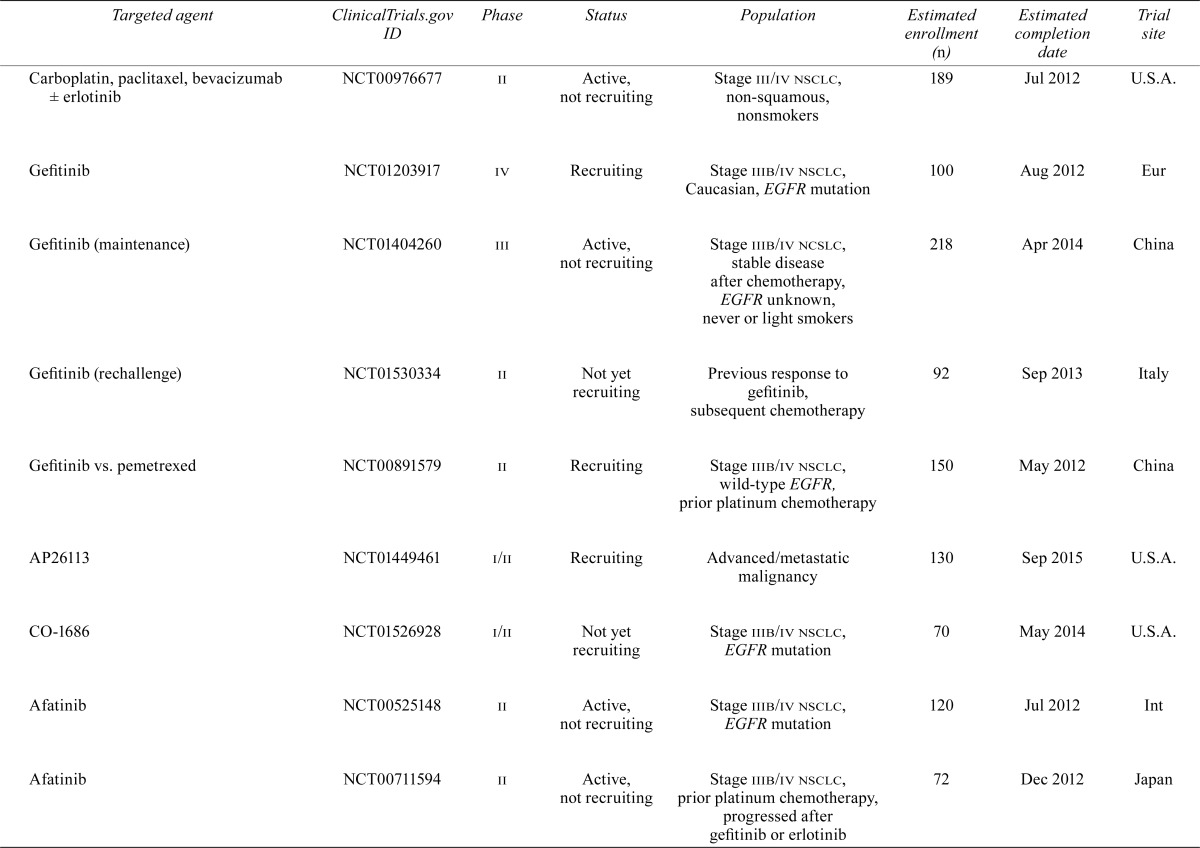
| Carboplatin, paclitaxel, bevacizumab ± erlotinib | NCT00976677 | ii | Active, not recruiting | Stage iii/iv nsclc, non-squamous, nonsmokers | 189 | Jul 2012 | U.S.A. |
| Gefitinib | NCT01203917 | iv | Recruiting | Stage iiib/iv nsclc, Caucasian, EGFR mutation | 100 | Aug 2012 | Eur |
| Gefitinib (maintenance) | NCT01404260 | iii | Active, not recruiting | Stage iiib/iv ncslc, stable disease after chemotherapy, EGFR unknown, never or light smokers | 218 | Apr 2014 | China |
| Gefitinib (rechallenge) | NCT01530334 | ii | Not yet recruiting | Previous response to gefitinib, subsequent chemotherapy | 92 | Sep 2013 | Italy |
| Gefitinib vs. pemetrexed | NCT00891579 | ii | Recruiting | Stage iiib/iv nsclc, wild-type EGFR, prior platinum chemotherapy | 150 | May 2012 | China |
| AP26113 | NCT01449461 | i/ii | Recruiting | Advanced/metastatic malignancy | 130 | Sep 2015 | U.S.A. |
| CO-1686 | NCT01526928 | i/ii | Not yet recruiting | Stage iiib/iv nsclc, EGFR mutation | 70 | May 2014 | U.S.A. |
| Afatinib | NCT00525148 | ii | Active, not recruiting | Stage iiib/iv nsclc, EGFR mutation | 120 | Jul 2012 | Int |
| Afatinib | NCT00711594 | ii | Active, not recruiting | Stage iiib/iv nsclc, prior platinum chemotherapy, progressed after gefitinib or erlotinib | 72 | Dec 2012 | Japan |
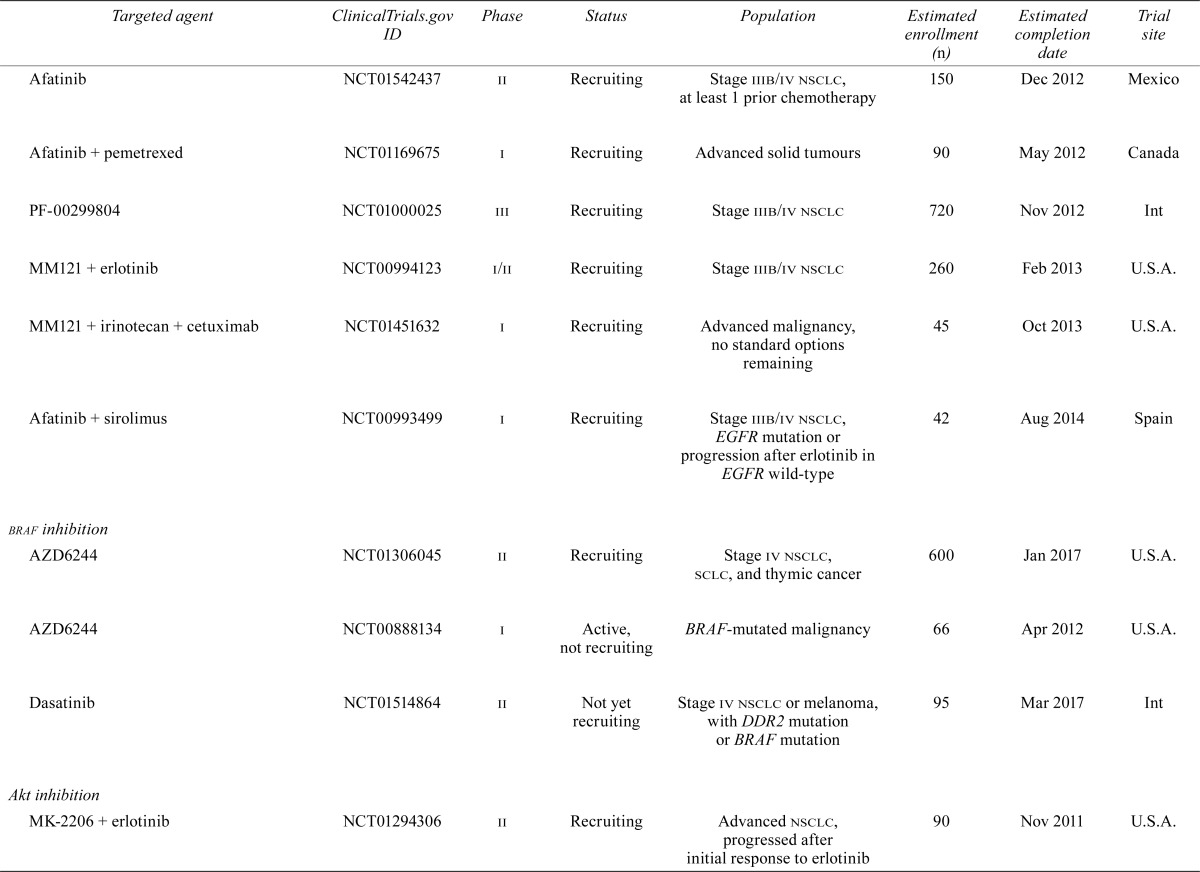
| Afatinib | NCT01542437 | ii | Recruiting | Stage iiib/iv nsclc, at least 1 prior chemotherapy | 150 | Dec 2012 | Mexico |
| Afatinib + pemetrexed | NCT01169675 | i | Recruiting | Advanced solid tumours | 90 | May 2012 | Canada |
| PF-00299804 | NCT01000025 | iii | Recruiting | Stage iiib/iv nsclc | 720 | Nov 2012 | Int |
| MM121 + erlotinib | NCT00994123 | i/ii | Recruiting | Stage iiib/iv nsclc | 260 | Feb 2013 | U.S.A. |
| MM121 + irinotecan + cetuximab | NCT01451632 | i | Recruiting | Advanced malignancy, no standard options remaining | 45 | Oct 2013 | U.S.A. |
| Afatinib + sirolimus | NCT00993499 | i | Recruiting | Stage iiib/iv nsclc, EGFR mutation or progression after erlotinib in EGFR wild-type | 42 | Aug 2014 | Spain |
| braf inhibition | |||||||
| AZD6244 | NCT01306045 | ii | Recruiting | Stage iv nsclc, sclc, and thymic cancer | 600 | Jan 2017 | U.S.A. |
| AZD6244 | NCT00888134 | i | Active, not recruiting | BRAF-mutated malignancy | 66 | Apr 2012 | U.S.A. |
| Dasatinib | NCT01514864 | ii | Not yet recruiting | Stage iv nsclc or melanoma, with DDR2 mutation or BRAF mutation | 95 | Mar 2017 | Int |
| Akt inhibition | |||||||
| MK-2206 + erlotinib | NCT01294306 | ii | Recruiting | Advanced nsclc, progressed after initial response to erlotinib | 90 | Nov 2011 | U.S.A. |
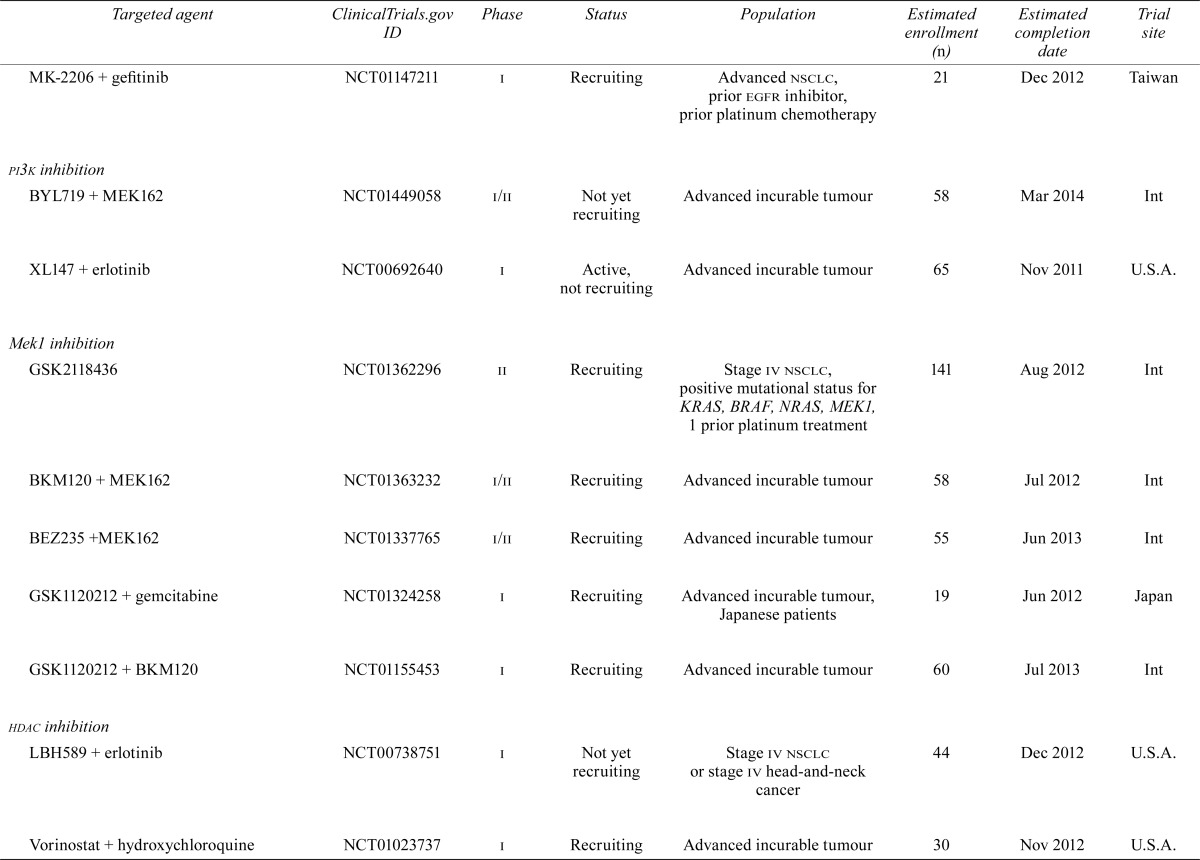
| MK-2206 + gefitinib | NCT01147211 | i | Recruiting | Advanced nsclc, prior egfr inhibitor, prior platinum chemotherapy | 21 | Dec 2012 | Taiwan |
| pi3k inhibition | |||||||
| BYL719 + MEK162 | NCT01449058 | i/ii | Not yet recruiting | Advanced incurable tumour | 58 | Mar 2014 | Int |
| XL147 + erlotinib | NCT00692640 | i | Active, not recruiting | Advanced incurable tumour | 65 | Nov 2011 | U.S.A. |
| Mek1 inhibition | |||||||
| GSK2118436 | NCT01362296 | ii | Recruiting | Stage iv nsclc, positive mutational status for KRAS, BRAF, NRAS, MEK1, 1 prior platinum treatment | 141 | Aug 2012 | Int |
| BKM120 + MEK162 | NCT01363232 | i/ii | Recruiting | Advanced incurable tumour | 58 | Jul 2012 | Int |
| BEZ235 +MEK162 | NCT01337765 | i/ii | Recruiting | Advanced incurable tumour | 55 | Jun 2013 | Int |
| GSK1120212 + gemcitabine | NCT01324258 | i | Recruiting | Advanced incurable tumour, Japanese patients | 19 | Jun 2012 | Japan |
| GSK1120212 + BKM120 | NCT01155453 | i | Recruiting | Advanced incurable tumour | 60 | Jul 2013 | Int |
| hdac inhibition | |||||||
| LBH589 + erlotinib | NCT00738751 | i | Not yet recruiting | Stage iv nsclc or stage iv head-and-neck cancer | 44 | Dec 2012 | U.S.A. |
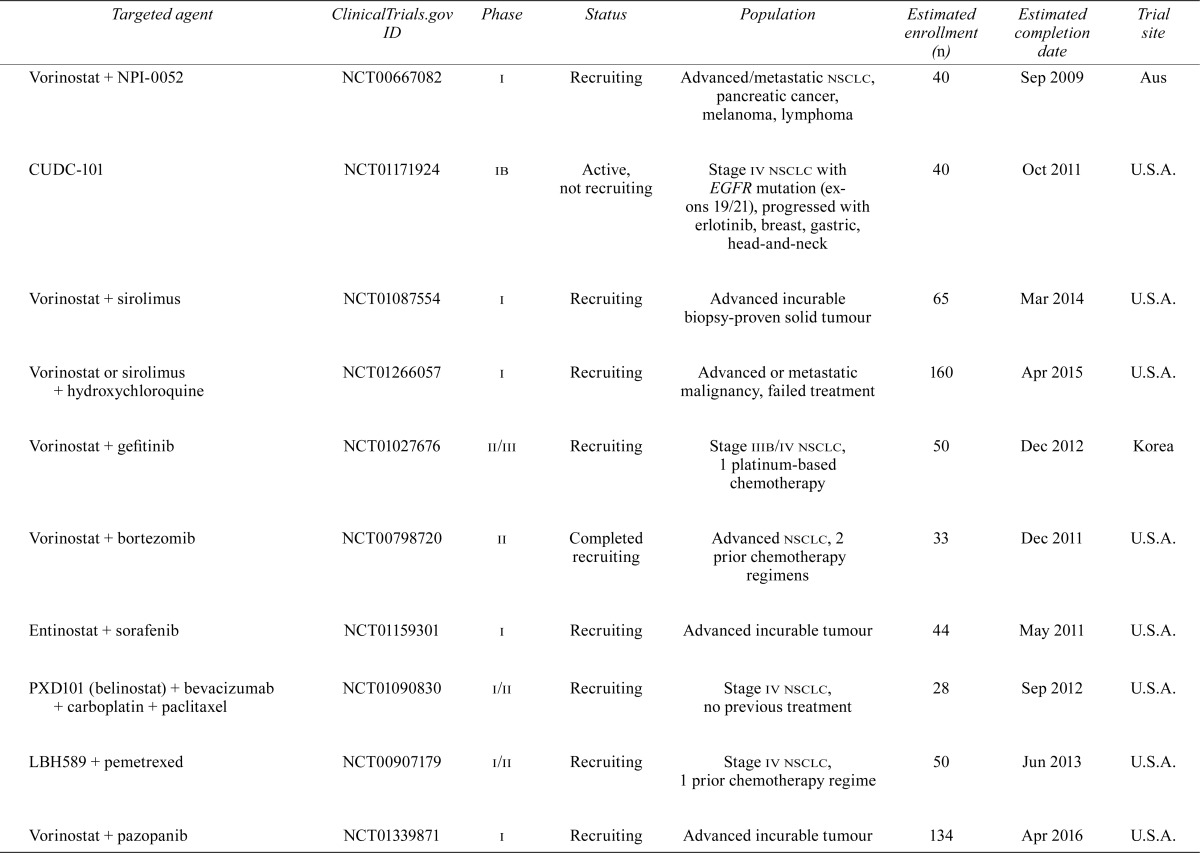
| Vorinostat + hydroxychloroquine | NCT01023737 | i | Recruiting | Advanced incurable tumour | 30 | Nov 2012 | U.S.A. |
| Vorinostat + NPI-0052 | NCT00667082 | i | Recruiting | Advanced/metastatic nsclc, pancreatic cancer, melanoma, lymphoma | 40 | Sep 2009 | Aus |
| CUDC-101 | NCT01171924 | ib | Active, not recruiting | Stage iv nsclc with EGFR mutation (exons 19/21), progressed with erlotinib, breast, gastric, head-and-neck | 40 | Oct 2011 | U.S.A. |
| Vorinostat + sirolimus | NCT01087554 | i | Recruiting | Advanced incurable biopsy-proven solid tumour | 65 | Mar 2014 | U.S.A. |
| Vorinostat or sirolimus + hydroxychloroquine | NCT01266057 | i | Recruiting | Advanced or metastatic malignancy, failed treatment | 160 | Apr 2015 | U.S.A. |
| Vorinostat + gefitinib | NCT01027676 | ii/iii | Recruiting | Stage iiib/iv nsclc, 1 platinum-based chemotherapy | 50 | Dec 2012 | Korea |
| Vorinostat + bortezomib | NCT00798720 | ii | Completed recruiting | Advanced nsclc, 2 prior chemotherapy regimens | 33 | Dec 2011 | U.S.A. |
| Entinostat + sorafenib | NCT01159301 | i | Recruiting | Advanced incurable tumour | 44 | May 2011 | U.S.A. |
| PXD101 (belinostat) + bevacizumab + carboplatin + paclitaxel | NCT01090830 | i/ii | Recruiting | Stage iv nsclc, no previous treatment | 28 | Sep 2012 | U.S.A. |
| LBH589 + pemetrexed | NCT00907179 | i/ii | Recruiting | Stage iv nsclc, 1 prior chemotherapy regime | 50 | Jun 2013 | U.S.A. |
| Vorinostat + pazopanib | NCT01339871 | i | Recruiting | Advanced incurable tumour | 134 | Apr 2016 | U.S.A. |
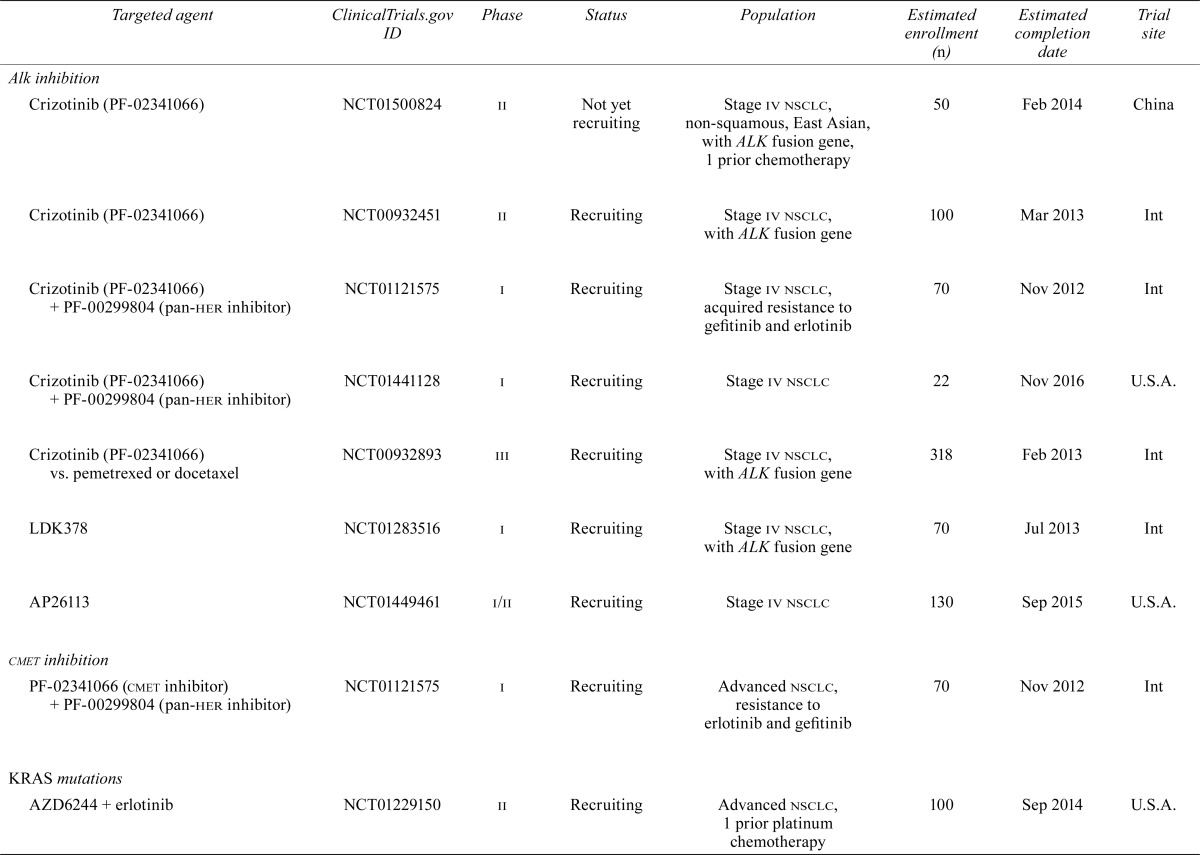
| Alkinhibition | |||||||
| Crizotinib (PF-02341066) | NCT01500824 | ii | Not yet recruiting | Stage iv nsclc, non-squamous, East Asian, with ALK fusion gene, 1 prior chemotherapy | 50 | Feb 2014 | China |
| Crizotinib (PF-02341066) | NCT00932451 | ii | Recruiting | Stage iv nsclc, with ALK fusion gene | 100 | Mar 2013 | Int |
| Crizotinib (PF-02341066) + PF-00299804 (pan-her inhibitor) | NCT01121575 | i | Recruiting | Stage iv nsclc, acquired resistance to gefitinib and erlotinib | 70 | Nov 2012 | Int |
| Crizotinib (PF-02341066) + PF-00299804 (pan-her inhibitor) | NCT01441128 | i | Recruiting | Stage iv nsclc | 22 | Nov 2016 | U.S.A. |
| Crizotinib (PF-02341066) vs. pemetrexed or docetaxel | NCT00932893 | iii | Recruiting | Stage iv nsclc, with ALK fusion gene | 318 | Feb 2013 | Int |
| LDK378 | NCT01283516 | i | Recruiting | Stage iv nsclc, with ALK fusion gene | 70 | Jul 2013 | Int |
| AP26113 | NCT01449461 | i/ii | Recruiting | Stage iv nsclc | 130 | Sep 2015 | U.S.A. |
| cmet inhibition | |||||||
| PF-02341066 (cmet inhibitor) + PF-00299804 (pan-her inhibitor) | NCT01121575 | i | Recruiting | Advanced nsclc, resistance to erlotinib and gefitinib | 70 | Nov 2012 | Int |
| KRAS mutations | |||||||
| AZD6244 + erlotinib | NCT01229150 | ii | Recruiting | Advanced nsclc, 1 prior platinum chemotherapy | 100 | Sep 2014 | U.S.A. |
| Erlotinib + ARQ 197 vs. single-agent chemotherapy | NCT01395758 | ii | Recruiting | Stage iv nsclc, with KRAS mutation | 98 | Jun 2012 | U.S.A. |
| GSK1120212 vs. docetaxel | NCT01362296 | ii | Recruiting | Stage iv nsclc, positive mutational status for KRAS, NRAS, BRAF, MEK1 | 141 | Aug 2012 | Int |
nsclc = non-small-cell lung cancer; egfr-tki = epidermal growth factor receptor tyrosine kinase inhibitor; Int = international (more than one continent); Eur = Europe; sclc = small-cell lung cancer; Aus = Australia; her = human epidermal growth factor receptor.