Abstract
Objectives/ Hypothesis
High mobility group box 1(HMGB1) protein has been identified as a principal instigator of injury-induced inflammation in many organ systems. Physiologically, HMGB1 binds to chromatin in cell nucleus. Upon injury, cells release HMGB1 to extracellular milieu triggering a destructive inflammatory response. Neutralizing or removing HMGB1 has been shown to control inflammation. Unfortunately, the role of HMGB1 in laryngeal inflammation and healing is yet defined. The purpose of this study was to determine spatial and temporal patterns of HMGB1 expression in surgically injured rat vocal folds up to 2 weeks post injury.
Study Design
Prospective animal study.
Methods
Bilateral vocal fold injury was performed on 70 Sprague-Dawley rats. An additional 14 rats served as uninjured controls. Animals were sacrificed at 1 day, 3 days, 5 days, 1 week, and 2 weeks following surgery. Imunohistochemistry staining and enzyme-linked immunosorbent assay (ELISA) was performed to determine the spatial distribution and temporal expression of HMGB1 in vocal fold tissue respectively. Hematoxylin and eosin staining for cell counting was performed to evaluate cell infiltration.
Results
Cell number peaked significantly at five days following injury. HMGB1 was positively stained in the nuclear, cytoplasmic and extracellular compartments from Days 1 to 7 after injury; whereas a strict nuclear staining was observed in uninjured controls and Week 2 animals. Staining results were corroborated by ELISA.
Conclusions
Spatial and temporal changes of HMGB1 expression were shown in injured vocal fold tissue, indicating this protein may be one of the principal drivers of inflammation and the healing response to surgical injury in the larynx.
Keywords: HMGB1, danger signals, vocal folds, inflammation, wound healing
INTRODUCTION
Inflammation is an innate immune response to noxious stimuli. The classical immunology model of self-non-self discrimination stipulates that the immune system does not react against the self but reacts against the non-self or foreign agents such as pathogenic bacteria, viruses, and parasites 1,2. In the vocal folds, surgical trauma or phonotrauma is rarely associated with bacterial infections but rather mechanical injury. The ensued scale of inflammation is important in mediating tissue repair mechanisms that determines the final healing outcome. In the absence of foreign invaders as in infection-induced inflammation, the principle purveyor of the injury-induced inflammation is from endogenous products released by distressed cells or tissues. Such endogenous molecules are known as Damage Associated Molecular Pattern (DAMP) molecules or “danger signals” that instigate injury-induced inflammation and healing3,4.
Chromatin-associated protein high-mobility group box 1 (HMGB1) is one of the DAMP molecules released by injured cells upon mechanical challenge 5–13. HMGB1 is a 30kDa chromatin-binding protein that is ubiquitously expressed in normal cells. In physiological conditions, HMGB1 is sequestered in cell nucleus to stabilize DNA structure 14 and thus is hidden from recognition by the immune system. Upon injury, however, necrotic cells release HMGB1 from the intracellular compartment to the extracellular milieu. When HMGB1 is exposed outside the cells, this protein become recognized by immune receptors and triggers various cellular responses pertinent to innate immunity and tissue repair. For example, immune receptors including toll-like receptor (TLR) 2, TLR4, TLR9 15 are activated by HMGB1, resulting an induction of multiple pro-inflammatory cytokines, such as tumor necrosis factors (TNF) 13,16–21 that sets the inflammation and healing process in motion.
The presence of HMGB1 has been well documented in various inflammatory diseases, such as arthritis and sepsis 6,15,22. Neutralizing or blocking HMGB1was shown to attenuate inflammation and reduce tissue damage 13. An anti-HMGB1 treatment of mice with liver necrosis ameliorated inflammatory cell recruitment 23. Identifying HMGB1 in mechanically injured vocal folds is fundamental to understanding the mechanism by which the inflammatory and healing response is initiated and may suggest a potential therapeutic target for inflammation control to optimize vocal fold healing. Unfortunately, no investigation has documented the presence and functions of HMGB1 in the vocal folds. The primary goal of this study was to investigate whether HMGB1 was secreted to extracellular compartments that might function as “danger signals” in the vocal folds upon surgical injury. The precise spatial and temporal patterns of HMGB1 expression during vocal fold inflammation and healing were characterized using immunohistochemistry (IHC) and enzyme-linked immunosorbent protein assay (ELISA).
MATERIALS AND METHODS
Rat Vocal Fold Surgery
The current animal study was approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison (protocol MO2358). Eighty-four Sprague-Dawley male rats, 4 to 6 months old (450 to 500 g), were used in the study. Vocal fold injuries were created by following an established protocol 24. Briefly, animals were anesthetized and their vocal folds were injured bilaterally using a 25-gauge needle to strip the vocal folds until the thyroarytenoid muscle was exposed. Fourteen rats with injured vocal folds were euthanized for laryngeal harvest at each of five post-surgery time points: 1 day, 3 days, 5 days, 1 week, and 4 weeks. An additional 14 animals had their larynges harvested as uninjured controls. Animals were euthanized via CO2 asphyxiation. Immediately following euthanasia, total laryngectomy was performed. For each time point, two of the 14 larynges were fixed in formalin for histological analysis. The remaining 12 larynges underwent micro-dissection procedures of vocal fold mucosa for protein analysis.
Immunohisochemistry of HMGB1 Localization
Formalin-fixed laryngeal specimens were embedded in paraffin and sectioned at 5 μm in the coronal plane. Only sections containing the mid-membranous vocal folds were subjected to IHC staining for HMGB1. A primary rabbit polyclonal antibody against HMGB1 protein in 1:1000 (Abcam, Cambridge, MA) and a secondary rabbit-on-rodent horseradish peroxidase micro-polymer (Biocare Medical, Concord, CA) were used. Diaminobenzidine chromogen (BD Biosciences, San Jose, CA) was used to detect horseradish peroxidase prior to counter staining with hematoxylin and mounting. Routine hematoxylin and eosin (H&E) staining was also performed to evaluate the overall vocal fold morphology over the time course. All stained sections were viewed with a Nikon E600 microscope (Nikon, Melville, NY) and were photographed using an Olympus DP71 microscope digital camera (Tokyo, Japan). In order to evaluate whether the change of the HMGB1 expression was potentially due to the change of cell number in the wound site, cell counting was performed in this study. One representative H&E picture of each mid-membraneous vocal fold for each of the two animals at 200X magnification was selected and the number of cells in the lamina propria was counted by two independent blinded raters and then averaged for analysis. Inter-rater reliability was at Pearson’s r = 0.986 (p<0.05).
Quantification of HMGB1 Proteins
Upon injury, HMGB1 proteins translocate from the cell nucleus to the cytoplasm and eventually to the extracellular milieu becoming “danger signals”. The presence of HMGB1 in the cytoplasmic compartment has been regarded a characteristic marker for active secretion of HMGB1 to the extracellular milieu 25. In order to evaluate the translocation process of HMGB1 in injured vocal folds, time-varying protein quantities in both nucleus and cytoplasmic compartments were tracked in the present study. For each larynx, both vocal fold mucosa was dissected from the thyroarytenoid muscle under a stereo dissection microscope. Based on pilot data, four pairs of vocal folds were necessary to give one workable testing sample for downstream protein analysis. Therefore, per each time point, 12 pairs of vocal fold mucosa were dissected to give three workable testing samples for cytoplasmic-nuclear protein extractions.
To extract cytoplasmic and nuclear proteins, testing samples were homogenized on ice, lysed and separated into nuclear and cytoplasmic fractions using the NE-PER nuclear and cytoplasmic extraction kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions. The supernatant was collected and its protein concentration was measured using micro BCA protein assay (Thermo Scientific, Rockford, IL) in duplicates. The microBCA data were used to normalized the downstream ELISA data for each corresponding sample.
HMGB1 ELISA kits from IBL International (Hamburg, Germany) were used to quantify HMGB1 proteins by following the manufacturer’s instructions. Briefly, 100 μl of diluent buffer were added to each well. Ten μl of standards, positive control and testing samples were added to each well and incubated for 24 hour at 37 °C. Wells were washed five times with wash buffer and incubated for 2 hour at 25 °C with 100 μl of peroxidase-conjugate solution to each well. Subsequently, wells were washed five times in wash buffer and incubated for 30 minutes at room temperature with 100 μl of color solution. 100 μl of stop solution was added to each well to stop the reaction. Wells were read at 450 nm on a FlexStation 3 microplate reader (Molecular Devices, Sunnyvale, USA). Experimental samples were run in triplicate.
Statistical Analysis
One-way analysis of variance (ANOVA) was used to test the differences in cell numbers and ELISA HMGB1 expressions across time. If F-tests revealed significant differences, post-hoc pairwise comparisons using Fisher’s protected least squares difference (LSD) were carried out. An α-level of 0.05 was employed for all comparisons. Unadjusted α levels were used because Type II (β) error as much as from Type I (α) error were opted to protect at this early stage of inquiry to predict the cell numbers and this novel protein expression in surgically injured vocal folds.
RESULTS
HMGB1 Spatial Localization in Injured Rat Vocal Folds
Uninjured vocal folds of control animals showed a strict nuclear localization of HMGB1 (Figure 1A). Starting at Day 1 post surgery, injured vocal folds revealed nuclear-to-cytoplasmic redistribution of HMGB1 (Figure 1C) as compared to uninjured controls (Figure 1A). Furthermore, massive cytoplasmic-to-extracellular deposition of HMGB1 was evident from Day 3 through Day 7 post surgery (Figure 1E, G and I), concomitantly with massive cellular infiltration and disorganized neo-matrix deposition (Figure 1F, H and J). At Day 14, HMGB1 staining returned to strict nuclear pattern as in the uninjured controls. Vocal fold re-epithelization was nearly completed and the neo-matrix was relatively organized for Day 14 animals (Figure 1K&L) compared to those from earlier surgical time points.
Figure 1. High-mobility group chromosomal box (HMGB1) expression following vocal fold surgical injury.
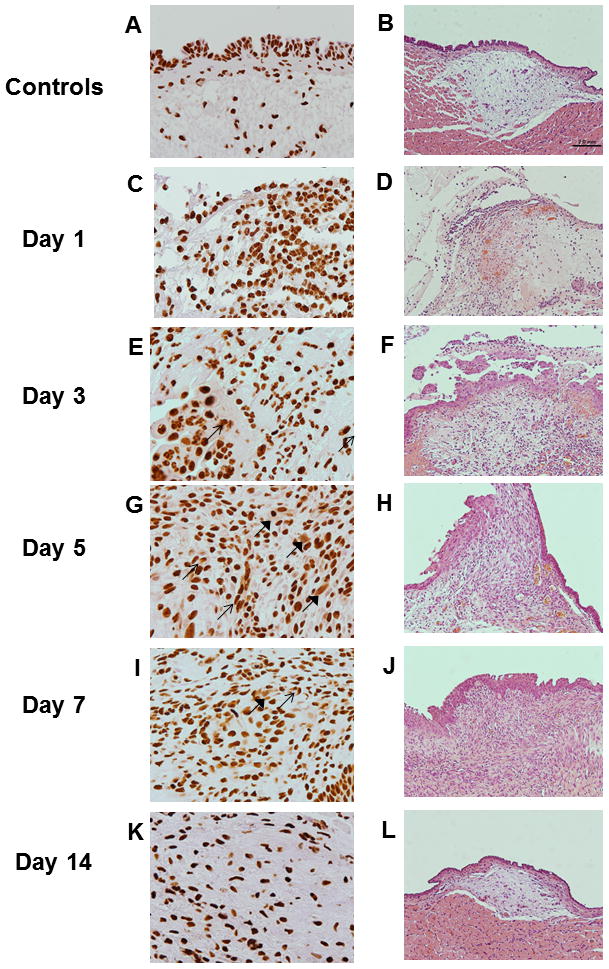
(A, C, E, F, I and K) Representative H&E staining of uninjured and injured vocal fold morphology over 2 week post surgery (200X magnification). (B, D, F, H, J and L) Representative IHC staining of HMGB1 of the same rats (600X magnification) in corresponding H&E micrographs. HMGB1 is stained brown. Positive nuclear staining was evident in uninjured vocal folds. In addition to the nuclear expression, cytoplasmic (thin arrows) and extracellular (thick arrows) HMGB 1 staining was evident in injured samples from Day 1 to Day 7 post surgery. In Day 14, cytoplasmic and extracellular HMGB1 deposition became less evident.
Time-varying Cell Numbers and HMGB1 Quantities in Injured Rat Vocal Folds
Compared to the uninjured controls, cell numbers were significant different overall (p≤0.05) across time points. Compared to uninjured controls, cell number increased significantly at 1 day (p = 0.04), 5 days (p = 0.01) and 7 days (p = 0.04) after injury. In particular cell number peaked at Day 5. Cell numbers were not significantly different between the uninjured controls and Day 14 animals (Figure 2).
Figure 2. Cell numbers in the lamina propria following vocal fold surgical injury.
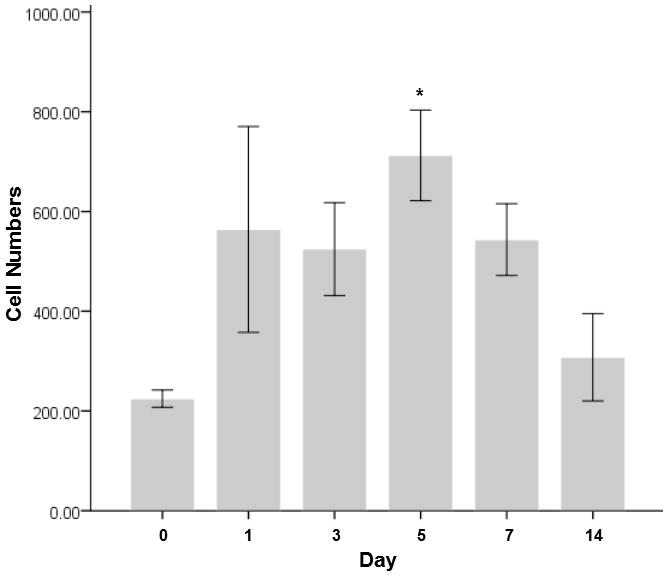
The bars and the error bars represent the mean and the standard errors of the data (n=12), representatively. Asterisks * denotes the data of that time point is statistically significant compared to the uninjured controls.
Per the ELISA HMGB1 data, both nuclear and cytoplasmic HMGB1 showed significant overall differences (p≤0.05) in expression across time points (Figure 3). Compared to uninjured controls, expressions of nuclear and cytoplasmic HMGB1 did not change significantly at 1 day post-injury. However, significantly increases in nuclear and cytoplasmic HMGB1 expressions were observed at 5 days (p = 0.02) and 3 days (p = 0.01) post-injury time points, respectively. Both nuclear and cytoplasmic HMGB1 was not significantly different from their controls at 7 and 14 days time points.
Figure 3. Nuclear and cytoplasmic HMGB1 protein levels normalized to the total protein level in the tissue samples following vocal fold surgical injury.
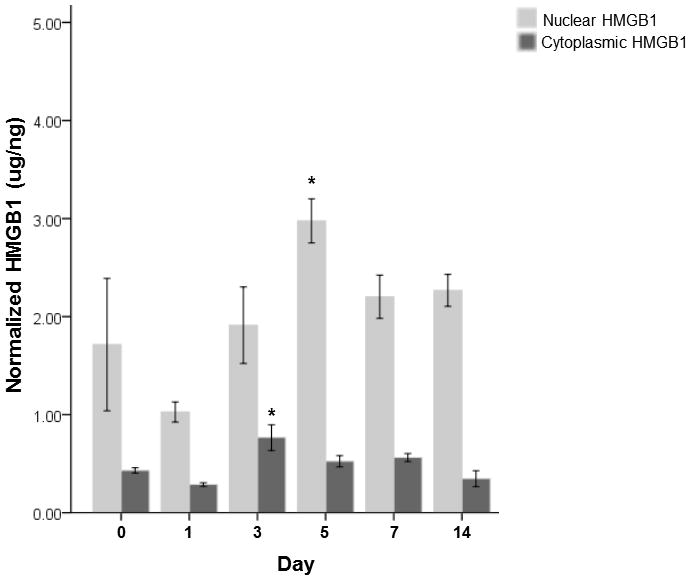
HMGB1 concentrations are in ug/ng. The bars and the error bars represent the mean and the standard errors of the data (n=18), representatively. Asterisks * denotes the data of that time point is statistically significant compared to the uninjured controls.
DISCUSSION
This study is the first to characterize the spatial and temporal expression of HMGB1 in the laryngeal system. HMGB1 is a nuclear protein known to be ubiquitously expressed in mammalian cells. In the present study, HMGB1 was positively expressed in vocal fold cell nuclei from both normal and injured tissue specimens, suggesting that our detection method was sensitive to visualize and quantify HMGB1 at both physiological and pathological levels. In injured vocal folds tissue samples, a dynamic HMGB1 expression was observed both spatially and quantitatively. Translocation of HMGB1 from the cell nucleus to the cytoplasm was noted early in the course of events, i.e., within the first 3 days after injury, suggesting the involvement of this protein in the early events of wound healing. The highest expression of cytoplasmic HMGB1 was found at 3 days post-surgery, which was coincided with the time window of active inflammation and extracellular deposition from the histological observations. These results suggested that HMGB1 proteins were released to extracellular milieu to function as “danger signals” during inflammation following vocal fold surgery.
In addition to the HMGB1 expression, the time-varying cell numbers within lamina propria were examined. The temporal pattern of the cell number from the current study corroborated with previous rat vocal fold reports 26,27. Furthermore, both nuclear HMGB1 level and cell count peaked at 5 days post injury. Peak expression of nuclear HMGB1 may be due to accumulation of HMGB1-nuclear-positive cells such as macrophages and fibroblasts in the wound site. On the other hand, the cytoplasmic HMGB1 level significantly increased and peaked at 3 days but then decreased significantly and returned to control levels at 5 days post-injury onwards. This temporal pattern of HMGB1 expression corroborated with mice skin wound healing literature 28. Our results suggested that nuclear-cytoplasmic translocation of HMGB1 was active between 1 day and 3 days in post-surgical vocal fold tissues. In contrast to the results of nuclear HMGB1, the peak of cytoplasmic HMGB1 came before the peak of the cell number, suggesting that the increase of the cytoplasmic HMGB1 level was primarily contributed by the nuclear-cytoplasmic translocation process activated by tissue injury. A high level of cytoplasmic HMGB1 indicates an active inflammatory activity ongoing in the vocal fold mucosal tissue. We propose that cytoplasmic HMGB1 may be a novel marker of the inflammatory conditions in the vocal folds.
HMGB1 can act as an early trigger or a late modulator of inflammation depending on the stimuli. When tissue is first injured, HMGB1 is passively released from the nuclei of necrotic cells to the extracellular space. Secreted HMGB1 activates inflammatory cells triggering a cascade of inflammatory responses. Besides responding to early tissue damage, HMGB1 is also actively released by native and infiltrating inflammatory cells in response to pro-inflammatory cytokines such as TNF-α, interleukin (IL)1-β albeit with a delay of 9–16 hours in mice after lipopolysaccharide-induced lethal endotoxemia 29. The secreted HMGB1further stimulates the release of pro-inflammatory cytokines and HMGB1 of the same cells in an autocrine manner.
If vocal fold HMGB1 exhibits the aforesaid dual function that both instigate (an early trigger) and sustain (a late modulator) the inflammation process, the temporal pattern of cytoplasmic HMGB1 expression should be seen as bimodal in our data. The first peak of cytoplasmic HMGB1 was seen at 3 days after injury. However, the second peak of cytoplasmic HMGB1 expression was obscure in our data. A slight rebound of cytoplasmic HMGB1 level was observed at 7 days post injury that was not statistically significant compared to the preceding day (5-day) and the uninjured controls. That said, comparing the current HMGB1 data with reported temporal profiles of vocal fold cytokines reported in the literature may provide insights of whether HMGB1 acted as an autocrine stimulus in vocal fold inflammation and healing. In previous rat vocal fold studies 30,31, mRNA expressions of both TNF-α and IL1-β were reported to be significantly increased at 1 hour, 4 hours and 8 hours compared to the uninjured controls. Our 3-day peak of cytoplasmic HMGB1 may be the active response to TNF-α and IL1-β in addition to the injurious stimulus itself. At the same time, if secreted HMGB1 becomes an autocrine factor to stimulate the production of pro-inflammatory cytokines, upregulated mRNA expressions of TNF-α and IL1-β should also be seen around or after the 3-day time point. However, mRNA expressions of both TNF-α and IL1-β were not reported to be significantly different at 3 days following injury 31, which was the furthest time-point from injury for gene expression levels investigated in the literature. Changes in protein levels have been reported in the literature for later time points. In a rabbit vocal fold study 32, the protein concentration of IL1-β peaked at Day 1 and appeared to rebound at Day 14. Our data also showed a similar rebound of cytoplasmic HMGB1 at 7 days post injury. Thus, if vocal fold HMGB1 acts as an autocrine stimulus to the release of pro-inflammatory cytokines, such action may take place between 7 and 14 days after injury.
The precise cell source of HMGB1in injured vocal fold is unknown. Activated macrophages and fibroblasts have been documented as the primary cells to release HMGB1 in response to injury in other parts of the body 13. Both cells were populated in native and injured vocal fold lamina propria 26,27,33. However, macrophages were reported to be minimally migrated or proliferated in injured rat vocal folds whereas fibroblasts were dominantly present over the time course of inflammation and healing 27. Considering that fibroblasts are the primary cells in both native and injured vocal fold lamina propria, we propose that fibroblasts might be one of the primary cell sources in secreting HMGB1 to modulate inflammation and wound repair in vocal fold microenvironment. Once the cellular origin of HMGB1 is confirmed, such cell types can be the targets for anti-HMGB1 treatments to attenuate inflammation and optimize healing following injury. Studies are in progress in our laboratory to define the cellular origins of HMGB1 in vocal fold tissue.
There is a limitation in this investigation that warrants discussion. The ELISA assay required at least 2.5 ng/ ml protein extraction for valid quantification. Because of the size of the rat’s vocal folds, tissue samples were pooled to provide sufficient protein qualities for downstream assaying. Four pairs of rat vocal folds were combined for each assay. Such tissue pooling reduced the sample size for each time point, limiting information on individual variation and decreased statistical power.
CONCLUSION
In surgically injured vocal folds, the endogeneous danger signal HMGB1 was translocated from the cell nuclei to the cytoplasm during the acute phase of wound healing. This dynamic HMGB1 expression indicates its potential role in inciting vocal fold inflammation and healing. Thus, HMGB1 may constitute a potential therapeutic target to control vocal fold inflammation following mechanical trauma. Further work is currently being undertaken to identify the cell source of this important molecule and its roles in the induction and propagation of vocal fold inflammation.
Acknowledgments
Source of Funding
The study was funded by the National Institute on Deafness and Other Communication Disorders (R01DC4336 and DC9600). The authors have no other funding, financial relationships, or conflicts of interest to disclose.
The authors would like to acknowledge Drew Roenneburg for the H&E staining, Glen Leverson for the statistical consultation, Fei Chen for the cell counting and Xia Chen for the general technical consultation of this project.
Footnotes
Level of evidence: N/A
References
- 1.Janeway C. Immunogenicity signals 1,2,3 ..and 0. Immunology today. 1989;10:283–286. doi: 10.1016/0167-5699(89)90081-9. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunology today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. The danger model: a renewed sense of self. Science (New York, NY. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 4.Kaczorowski DJ, Mollen KP, Edmonds R, Billiar TR. Early events in the recognition of danger signals after tissue injury. Journal of leukocyte biology. 2008;83:546–552. doi: 10.1189/jlb.0607374. [DOI] [PubMed] [Google Scholar]
- 5.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Molecular medicine (Cambridge, Mass. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foell D, Wittkowski H, Roth J. Mechanisms of disease: a 'DAMP' view of inflammatory arthritis. Nature clinical practice. 2007;3:382–390. doi: 10.1038/ncprheum0531. [DOI] [PubMed] [Google Scholar]
- 7.Palumbo R, Sampaolesi M, De Marchis F, et al. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. The Journal of cell biology. 2004;164:441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. Journal of internal medicine. 2004;255:320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Tracey KJ. High mobility group box 1 (HMGB1) Critical care medicine. 2005;33:S472–474. doi: 10.1097/01.ccm.0000187005.81616.a9. [DOI] [PubMed] [Google Scholar]
- 10.van Zoelen MA, Ishizaka A, Wolthuls EK, Choi G, van der Poll T, Schultz MJ. Pulmonary levels of high-mobility group box 1 during mechanical ventilation and ventilator-associated pneumonia. Shock. 2008;29:441–445. doi: 10.1097/SHK.0b013e318157eddd. [DOI] [PubMed] [Google Scholar]
- 11.Peltz ED, Moore EE, Eckels PC, et al. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32:17–22. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peltz ED, Moore EE, Eckels PC, et al. Hmgb1 Is Markedly Elevated within Six Hours of Mechanical Trauma in Humans. Shock. 2008 doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson U, Tracey KJ. HMGB1 Is a Therapeutic Target for Sterile Inflammation and Infection. Annual review of immunology. 2010 doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta. 2010;1799:101–113. doi: 10.1016/j.bbagrm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nature reviews. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. The Journal of experimental medicine. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMarco RA, Fink MP, Lotze MT. Monocytes promote natural killer cell interferon gamma production in response to the endogenous danger signal HMGB1. Molecular immunology. 2005;42:433–444. doi: 10.1016/j.molimm.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Kokkola R, Andersson A, Mullins G, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scandinavian journal of immunology. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 19.Orlova VV, Choi EY, Xie C, et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. The EMBO journal. 2007;26:1129–1139. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren D, Sun R, Wang S. Role of inducible nitric oxide synthase expressed by alveolar macrophages in high mobility group box 1--induced acute lung injury. Inflamm Res. 2006;55:207–215. doi: 10.1007/s00011-006-0072-2. [DOI] [PubMed] [Google Scholar]
- 21.Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin) Journal of leukocyte biology. 2007;81:49–58. doi: 10.1189/jlb.0306200. [DOI] [PubMed] [Google Scholar]
- 22.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends in immunology. 2007;28:429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 24.Welham NV, Montequin DW, Tateya I, Tateya T, Choi SH, Bless DM. A rat excised larynx model of vocal fold scar. J Speech Lang Hear Res. 2009;52:1008–1020. doi: 10.1044/1092-4388(2009/08-0049). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Yin H, Han J, et al. Extracellular hmgb1 functions as an innate immune-mediator implicated in murine cardiac allograft acute rejection. Am J Transplant. 2007;7:799–808. doi: 10.1111/j.1600-6143.2007.01734.x. [DOI] [PubMed] [Google Scholar]
- 26.Ling C, Yamashita M, Waselchuk EA, Raasch JL, Bless DM, Welham NV. Alteration in cellular morphology, density and distribution in rat vocal fold mucosa following injury. Wound Repair Regen. 2010;18:89–97. doi: 10.1111/j.1524-475X.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tateya I, Tateya T, Lim X, Sohn JH, Bless DM. Cell production in injured vocal folds: a rat study. The Annals of otology, rhinology, and laryngology. 2006;115:135–143. doi: 10.1177/000348940611500210. [DOI] [PubMed] [Google Scholar]
- 28.Straino S, Di Carlo A, Mangoni A, et al. High-mobility group box 1 protein in human and murine skin: involvement in wound healing. The Journal of investigative dermatology. 2008;128:1545–1553. doi: 10.1038/sj.jid.5701212. [DOI] [PubMed] [Google Scholar]
- 29.Muller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. Journal of internal medicine. 2004;255:332–343. doi: 10.1111/j.1365-2796.2003.01296.x. [DOI] [PubMed] [Google Scholar]
- 30.Welham NV, Lim X, Tateya I, Bless DM. Inflammatory factor profiles one hour following vocal fold injury. The Annals of otology, rhinology, and laryngology. 2008;117:145–152. doi: 10.1177/000348940811700213. [DOI] [PubMed] [Google Scholar]
- 31.Lim X, Tateya I, Tateya T, Munoz-Del-Rio A, Bless DM. Immediate inflammatory response and scar formation in wounded vocal folds. The Annals of otology, rhinology, and laryngology. 2006;115:921–929. doi: 10.1177/000348940611501212. [DOI] [PubMed] [Google Scholar]
- 32.Branski RC, Rosen CA, Hebda PA, Verdolini K. Cytokine analysis of acute wound healing in the larynx: A rabbit model. The Voice Foundation's 32 Annual Symposim: Care of the Professional Voice; Philadelphia, PA. 2003. [Google Scholar]
- 33.Catten M, Gray SD, Hammond TH, Zhou R, Hammond EH. Analysis of cellular location and concentration in vocal fold lamina propria. Otolaryngology Head and Neck Surgery. 1998;118:663–667. doi: 10.1177/019459989811800516. [DOI] [PubMed] [Google Scholar]


