Fig. 9.
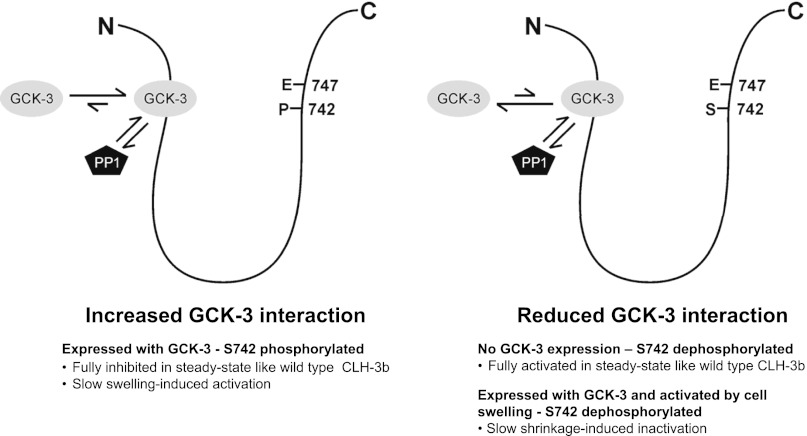
Working model of phosphorylation-dependent regulation of S747E mutant CLH-3b channels. Model assumes that GCK-3 and type 1 protein phosphatase (PP1) must compete for access to S742 and S747. Under steady-state conditions, S742 is fully phosphorylated and interaction of GCK-3 with the channel is high. This increased interaction slows swelling-induced channel dephosphorylation and associated channel activation (Fig. 2). When coexpressed with wild-type channels, the S747E linker functions as a sink for GCK-3, resulting in increased basal channel activity (Fig. 6, A–C). GCK-3 interaction is reduced by dephosphorylation of S742 in the S747E mutant. Reduced interaction slows shrinkage-induced inactivation of S747E mutant channels (Fig. 4). Dephosphorylation of the S747E mutant linker reduces its ability to interact with GCK-3, resulting in more rapid shrinkage-induced inactivation of coexpressed wild-type channels (Fig. 6E).