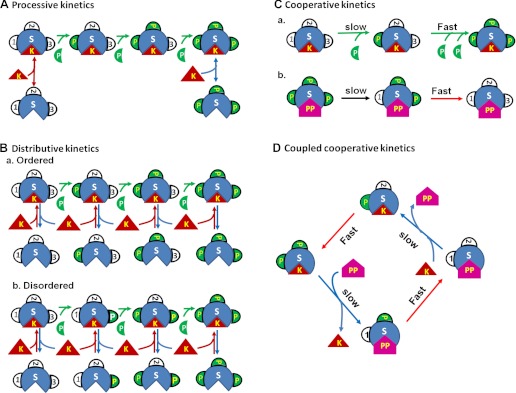
Fig. 1.
Distinct kinetic mechanisms of multisite protein phosphorylation (5, 7). A: processive kinetics: a single binding of the kinase (K) to the substrate (S) phosphorylates all sites of the substrate (full phosphorylation). B: distributive kinetics: multiple binding events are required for full phosphorylation of the substrate. The phosphorylation sites may be altered in a specific sequence (ordered, a) or in a random fashion (disordered, b). C: cooperative kinetics: the initial binding of kinase or phosphatase (PP) to the substrate not only causes initial phosphorylation (a) or dephosphorylation (b) of one site but also promotes phosphorylation or dephosphorylation of the other phosphorylation sites of the substrate. D: the coupled cooperative (or “double key”) kinetics. The phosphorylation of the two sites is closely coupled with the dephosphorylation process. When both sites are phosphorylated, it prevents phosphatase from replacing the kinase and binding to the substrates and slows down the dephosphorylation kinetics. The successful binding of the phosphatase to the substrate causes full dephosphorylation and also prevents the kinase from replacing the phosphatase and binding to the substrate in either a competitive (5) or noncompetitive manner, and thus, slows down the kinetics of phosphorylation of the first site.