Abstract
In several tissues, transient receptor potential vanilloid 4 (TRPV4) channels are involved in the response to hyposmotic challenge. Here we report TRPV4 protein in porcine lens epithelium and show that TRPV4 activation is an important step in the response of the lens to hyposmotic stress. Hyposmotic solution (200 mosM) elicited ATP release from intact lenses and TRPV4 antagonists HC 067047 and RN 1734 prevented the release. In isosmotic solution, the TRPV4 agonist GSK1016790A (GSK) elicited ATP release. When propidium iodide (PI) (MW 668) was present in the bathing medium, GSK and hyposmotic solution both increased PI entry into the epithelium of intact lenses. Increased PI uptake and ATP release in response to GSK and hyposmotic solution were abolished by a mixture of agents that block connexin and pannexin hemichannels, 18α-glycyrrhetinic acid and probenecid. Increased Na-K-ATPase activity occurred in the epithelium of lenses exposed to GSK and 18α-glycyrrhetinic acid + probenecid prevented the response. Hyposmotic solution caused activation of Src family kinase and increased Na-K-ATPase activity in the lens epithelium and TRPV4 antagonists prevented the response. Ionomycin, which is known to increase cytoplasmic calcium, elicited ATP release, the magnitude of which was no greater when lenses were exposed simultaneously to ionomycin and hyposmotic solution. Ionomycin-induced ATP release was significantly reduced in calcium-free medium. TRPV4-mediated calcium entry was examined in Fura-2-loaded cultured lens epithelium. Hyposmotic solution and GSK both increased cytoplasmic calcium that was prevented by TRPV4 antagonists. The cytoplasmic calcium rise in response to hyposmotic solution or GSK was abolished when calcium was removed from the bathing solution. The findings are consistent with hyposmotic shock-induced TRPV4 channel activation which triggers hemichannel-mediated ATP release. The results point to TRPV4-mediated calcium entry that causes a cytoplasmic calcium increase which is an essential early step in the mechanism used by the lens to sense and respond to hyposmotic stress.
Keywords: connexin and pannexin, Src family kinase, TRPV4 channel
the transient receptor potential vanilloid 4 (TRPV4) channel is expressed in a wide range of cell types (11, 27). The TRP channel family, of which TRPV4 is a member, is part of the sensory apparatus that enables cells to respond to a wide variety of stimuli (4). According to studies in various tissues, TRPV4 channels are able to respond to a remarkable variety of different perturbations including heat, mechanical, osmotic, and chemical stimuli (10). Although it may interact directly with the cytoskeleton (15) and while it is a nonselective cation channel, most of the reported cellular responses to TRPV4 activation stem from the entry of extracellular calcium ions (12, 24–27). In a wide variety of tissues, hyposmotic shock has been found to activate TRPV4 channel-mediated calcium entry (13, 33, 37). Although other mechanical stimuli activate TRPV4 channels, their responsiveness to cell swelling led TRPV4 to be thought of as a volume-regulated cation channel (4). In a human keratocyte cell line, TRPV4 channel has been shown to play an important role in the regulatory volume decrease in response to osmotic swelling and this functional response was accompanied with a strong Ca2+ influx (2). Here we report evidence that inhibition of TRPV4 channels abolishes a number of lens responses to hyposmotic shock.
Most lens cells, the lens fibers, lack mitochondria, endoplasmic reticulum, and nuclei (8). As a consequence, a single layer of epithelium situated on the anterior surface plays a critical role in ionic and osmotic homeostasis of the entire structure. When such homeostasis fails, cataract occurs. Transport mechanisms, like Na-K-ATPase, in the epithelium may need to sense and respond to events that occur in the lens fiber mass. Active Na+-K+ transport by the epithelium contributes to the maintenance of sodium and potassium concentrations throughout the lens fiber mass and Na-K-ATPase-specific activity is higher in the epithelium than elsewhere in the lens (35). We reported recently that exposure of the intact porcine lens to hyposmotic solution causes an increase of Na-K-ATPase activity in the epithelium (32). Andersson and coworkers (1) proposed that the transient increase of Na-K-ATPase activity that occurs in cells subjected to hyposmotic solution plays an important role in the regulatory volume decrease (RVD) process cells use to recover from swelling. In an intact lens, the epithelium may be stressed directly by the hyposmotic solution as well as indirectly by mechanical strain due to water entry into the fiber mass that causes swelling of the entire structure. An understanding of the response of the lens to hyposmotic stress is important in regard to diabetic cataract in which aldose reductase-mediated accumulation of sorbitol occurs in persons with poorly controlled blood sugar. Because of sorbitol accumulation, the difference in osmolarity between the inside and outside of the lens causes water entry, swelling of the fiber mass, and loss of lens transparency (28, 29).
The Na-K-ATPase activity response to hyposmotic shock is associated with ATP release from the lens and stimulation of the purinergic receptor (32). Interestingly, TRPV4 activation has been reported to lead to ATP release from esophageal keratinocytes by a mechanism that involves a vesicular ATP transporter, VNUT (24). In the lens, however, two lines of evidence suggested the mechanism responsible for ATP release involves connexin and pannexin hemichannels. First, 18α-glycyrrhetinic acid and probenecid inhibit ATP release by lenses subjected to hyposmotic solution. Second, exposure of the lens to hyposmotic solution increases the ability of a hemichannel probe, propidium iodide (PI) (MW 668), to enter the lens epithelium. We also demonstrated expression of connexin 43, connexin 50 and pannexin 1 proteins in the lens epithelium (32). In the present study we demonstrate expression of TRPV4 in the lens epithelium and examined the influence of TRPV4 on hemichannel-mediated ATP release from the lenses subjected to hyposmotic shock.
MATERIALS AND METHODS
Reagents.
GSK1016790A, 18α-glycyrrhetinic acid, probenecid, PI, EGTA, and DMSO were purchased from Sigma (St. Louis, MO). Ionomycin calcium salt, RN 1734, and HC 067047 were purchased from Tocris Biosciences (Ellisville, MO). All other chemicals, including those for preparing the Krebs solution, were of analytical grade and purchased from Sigma. Depending on their solubility, test agents were dissolved in water or in DMSO from freshly opened ampules. Rabbit anti-TRPV4 and TRPV4 blocking peptide (871 amino acid peptide derived from residues 850 to the COOH terminus of mouse TRPV4) were purchased from Abcam (Cambridge, MA). Rabbit anti-phosphotyrosine 416 SFK (pY416 SFK) and rabbit anti-β-actin polyclonal antibodies were obtained from Cell Signaling Technology (Danvers, MA). Goat anti-rabbit secondary antibody conjugated with IRDye 680 was purchased from LICOR (Lincoln, NE).
Lenses.
Adult porcine eyes were shipped on ice overnight from Hatfield Quality Meats (Philadelphia, PA) or were collected on the day of slaughter from the Meat Science Laboratory of the University of Arizona or from West Valley Meat Processing (Buckeye, AZ). Research use of porcine eyes conformed to the Federation of American Societies for Experimental Biology (FASEB) Statement of Principles for the Use of Animals in Research and Education and was approved by the University of Arizona Institutional Animal Care and Use Committee. The eyes were dissected open from the posterior, the suspensory ligaments that hold the lens were cut, and the intact lens was removed and placed in Krebs solution at 37°C. Before use, lenses were allowed to equilibrate 60 min in Krebs solution at 37°C and 5% CO2 in a humidified incubator.
Krebs solution.
Krebs solution (300 mosM) contained (in mM) 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 25 NaHCO3, 2.5 CaCl2, 1 MgCl2, and 5.5 glucose. In hyposmotic Krebs solution (200 mosM), NaCl was reduced to 69 mM. Osmolarity was measured using a micro osmometer (μ Osmette, Precision System, Florence, AL) and adjusted with sucrose when necessary. Before use, the solution was equilibrated with 5% CO2 and adjusted to pH 7.4.
Measurement of ATP.
Intact lenses were each incubated in a separate well of a 24-well culture plate (Corning, Costar) which contained 1.3 ml Krebs solution. At the end of the equilibration period, the Krebs solution was gently removed using a Pasteur pipette and replaced with 1.3 ml of preincubation solution (37°C), added by gently trickling down the side of the well. In specified experiments the preincubation solution (Krebs solution, 300 mosM) contained test reagents such as RN 1734 or HC 067047. Unless otherwise specified, lenses were preincubated with the test reagents for 20 min. After this, the preincubation solution was removed and replaced with control Krebs solution or hyposmotic Krebs solution with or without test reagents. After 10 min, 100 μl of the incubation solution was collected, frozen in liquid nitrogen, and stored at −80°C. ATP in the solution was measured using a luciferin-luciferase bioluminescence assay kit (Molecular Probes, Eugene, OR), quantifying luminescence with a plate reader (Victor3 V, Perkin Elmer, San Jose, CA). Standard curves were obtained using solutions of ATP dissolved either in normal Krebs solution or in hyposmotic Krebs solution. The lyophilized luciferin-luciferase reagent, obtained from Sigma, was also dissolved in normal Krebs solution or in hypotonic Krebs solution. Two standard curves were necessary because low ionic strength and, to a lesser extent, osmolarity, influenced luminescence. The ATP concentration in the bathing solution, as a measure of ATP release by intact lenses, was calculated as nanomoles per lens. Because ATP release was slightly variable between batches of eyes, results from different experiments were not pooled.
PI uptake.
Following 20 min of preincubation in Krebs solution with or without test agents, intact lenses were incubated for 10 min in hyposmotic Krebs solution containing 25 μM PI (MW = 668.4) in the continued presence of the test agents. Lenses were then washed three times in control Krebs solution, and the capsule-epithelium was immediately removed and homogenized for 1 min (4 strokes of 15 s at 5-s intervals) using Misonix S3000 sonicator at a 6 W power setting (Misonix, Farmingdale, NY) in 300 μl of distilled water. The homogenate was passed through a 35-μm nylon mesh cell strainer (BD Falcon, catalog no. 352235) using low-speed centrifugation (2500 rpm, 1,000 g) for 5 min. As described previously (32), the cell strainer step was performed to exclude broken pieces of lens capsule material, which interfere with fluorescence reading. Protein in the sample was measured by bicinchoninic acid assay (34), and PI was measured by fluorescence at excitation and emission wavelengths of 535 nm and 617 nm, respectively, using Varioskan flash multimode reader (Thermo Scientific, Barrington, IL). The results are expressed as relative fluorescence/mg protein.
Na-K-ATPase activity.
Following 20 min of preincubation in Krebs solution with or without test agents, intact lenses were incubated for 10 min in hyposmotic solution in the continued presence of the test agents. Then the capsule-epithelium was removed, frozen in liquid nitrogen, and stored at −80°C until further processing. The capsule-epithelium was homogenized for 1 min (4 strokes of 15 s at 5-s intervals) using Misonix S3000 sonicator at a 6 W power setting in 400 μl of ice-cold 2×-strength ATPase assay buffer containing (in mM): 80 l-histidine, 200 NaCl, 10 KCl, 6.0 MgCl2, 2.0 EGTA (pH 7.4), and a protease inhibitor mixture at the manufacturer's recommended concentration (Roche Applied Science, Mannheim, Germany). The homogenate was subjected to centrifugation at 13,000 g for 25 min at 4°C to remove cell nuclei, larger mitochondria, and unbroken debris, then the supernatant was used to measure Na-K-ATPase activity. Homogenizing frozen samples in 2×-strength ATPase buffer enabled the ATPase reaction to be started by adding ATP in distilled water (see below). Control and experimental samples were treated in the same way. Protein in the sample was measured by the bicinchoninic acid assay (34) (Pierce Biotechnology, Rockford, IL), using bovine serum albumin as a standard.
Na-K-ATPase activity was measured using a slightly modified method described earlier (31). Samples obtained from treated or control lens epithelium (150 μl) were placed in tubes containing 50 μl of 2×-strength ice-cold Na-K-ATPase buffer. To improve access of ions and ATP to membrane vesicles, alamethicin solution in ethanol (5 μl) was added to give a final approximate concentration of 0.1 mg of alamethicin per mg protein (40). Ouabain, a specific Na-K-ATPase inhibitor (39), was added to half the tubes (final concentration 150 μM), and the remaining tubes received an equivalent volume (5 μl) of distilled water. An additional 150 μl of distilled water was added to each tube. The tubes were preincubated at 37°C for 5 min, then ATP (40 μl) was added to each tube (final concentration 2 mM), bringing the total assay mixture volume to 400 μl and the concentration of the 2×-strength Na-K-ATPase buffer to single-strength. After 30 min, the ATP hydrolysis reaction was stopped by the addition of 150 μl of 20% ice-cold trichloroacetic acid and placing the tubes on ice for 30 min with occasional shaking.
ATP hydrolysis was determined as the amount of inorganic phosphate released in each reaction tube. To detect inorganic phosphate, each tube was placed in a centrifuge at 3,000 rpm (2,680 g) for 15 min at 4°C, then 400 μl of the supernatant was removed and mixed with 400 μl of 4.0% FeSO4 solution in ammonium molybdate (1.25 g of ammonium molybdate in 100 ml of 2.5 N sulfuric acid). Standard solutions containing NaH2PO4 equivalent to 0, 10, 62.5, 125, 250, and 500 nmol PO4 were treated similarly. After 5 min at room temperature the tubes with the samples (not the standards) were placed in a centrifuge at 3,000 rpm for 10 min to pellet additional precipitates. Two hundred fifty microliters of each standard or sample were then transferred to each well of a 96-well plate and the absorbance was measured at 750 nm in a Perkin Elmer plate reader (Victor V3, Perkin Elmer). Na-K-ATPase activity was calculated as the difference between ATP hydrolysis in the presence and in the absence of ouabain. Values are presented as nmol ATP hydrolyzed per milligram protein per 30 min. Because Na-K-ATPase activity was variable between batches of eyes, data for different experiments were not pooled.
Western blot analysis.
Following 20 min of preincubation in Krebs solution with or without test agents, intact lenses were incubated for 2 min in hyposmotic Krebs solution in the continued presence of the test agents. Then the capsule-epithelium was removed and samples from two lenses were pooled and homogenized in 400 μl ice-cold lysis buffer that contained (in mM) 50 HEPES, 150 NaCl, 1 EDTA, 10 sodium fluoride, 10 sodium pyrophosphate, 2 sodium orthovanadate, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, and protease inhibitor cocktail at the manufacturer's recommended concentration (Roche Applied Science) (pH of 7.5). Homogenization was carried out for 1 min (4 strokes of 15 s at 5-s intervals) using a Misonix S3000 sonicator at 6W power setting. The homogenate was placed in a centrifuge at 13,000 g for 25 min at 4°C to remove nuclei, larger mitochondria, and unbroken debris. Protein was measured by bicinchoninic acid assay (34). The supernatant was subjected to Western blot analysis. Proteins were separated by 7.5% SDS-PAGE electrophoresis and transferred to nitrocellulose membrane. The membrane was kept overnight at 4°C in blocking buffer (LICOR) then incubated overnight at 4°C with one or more of the following: rabbit anti-TRPV4 polyclonal antibody (1 μg/ml); rabbit anti- pY416 SFK polyclonal antibody (1:1,000) and rabbit anti-β-actin polyclonal antibody (1:5,000). Where applicable, the primary antibody was first incubated at room temperature for 1 h with the specific blocking peptide appropriately diluted in the blocking buffer. After three 5-min washes with a mixture of Tris-buffered saline and Tween 20, the nitrocellulose membrane was incubated for 60 to 90 min with goat anti-rabbit secondary antibody conjugated with IRDye 680 (LICOR). Then the membrane was washed three times with Tris-buffered saline plus Tween 20 and three times with PBS. Immunoreactive bands were detected and quantified using an Odyssey infrared scanner (LICOR).
Cell culture.
A method of isolation and culture of epithelium from lenses of adult pig has been established. After removal of extraocular muscles and fat, the eyes were washed with HBSS containing 200 U/ml penicillin and 200 μg/ml streptomycin. Intact lenses were isolated as described earlier (32) but under sterile condition in a laminar flow cabinet. The eyes were dissected open from the posterior pole, the vitreous was removed by gentle pushing aside with a curved forceps, zonules were cut, and intact lenses were isolated. Any remnants of ciliary epithelium or vitreous were removed by rolling the lens over sterile filter paper. The lens was then placed on a petri dish with its posterior (more convex surface adjacent to the vitreous) face upward. The capsule-epithelium was isolated by making a small incision on the posterior capsule with a surgical blade and by holding and pulling apart the two cut margins using fine forceps. Care was taken to prevent curling or twisting of the capsule during isolation since the basement membrane of the lens epithelium is attached to the inner surface of the anterior capsule. Several capsule-epithelia (8) were placed on a large (100-mm diameter) petri dish with the cell side facing upward. A small amount of complete medium (1.0 ml) was added along the edge of the petri dish to prevent dehydration. The complete medium consists of a mixture of epithelial culture medium (EpiCM, ScienCell, catalog no. 4101), 2.0% fetal bovine serum, epithelial cell growth factor (1.0 ml/100 ml medium, ScienCell catalog no. 4152), and 100 U/ml penicillin plus 100 μg/ml streptomycin. The petri dish was then placed in a 37°C incubator with a humidified atmosphere of 95% air and 5% CO2 and incubated for 1 h to encourage firm attachment of the capsule with the plastic surface. Complete medium (5.0 ml) was then added carefully to the petri dish and incubated for 48 h. Medium was changed every alternate day after that. When a sufficient number of cells had spread out of the explants and before reaching over confluence at the epicenter (usually takes 6–8 days), cells were trypsinized for 3–5 min using 5 ml 1× trypsin EDTA (Gibco). Trypsin was immediately neutralized using 4 ml of a mixture of newborn calf serum and FBS (1:1). The cell-capsule suspension was separated from the capsule fragments by spinning at low speed (350 rpm, ∼20 g) for 5 min at 4°C and collecting the suspension from the top with a Pasteur pipette. The cell suspension was centrifuged at 1,000 rpm (168 g) for 10 min at 4°C, the supernatant was discarded, and the cells were seeded in a 25-cm2 flask at a density of 2 × 104 cells/cm2 in 5 ml of complete medium. Medium was changed after 24 h and then every alternate day. Cells usually reached confluence in 3–4 days. Cells were then trypsinized and passaged for maintenance and propagation. Only cells from passage 3 were used in this study. Cells cultured in this way retained epithelial characteristics as shown by E-cadherin and N-cadherin expression profiles determined by confocal immunolocalization (data not shown).
Measurement of cytoplasmic Ca2+.
Cytoplasmic calcium in cultured pig lens epithelium was measured in cells loaded with Fura-2 AM by measuring the fluorescence intensity at alternating excitation wavelengths of 340 nm and 380 nm, and the emission was captured at 510 nm using a modified method described earlier (23). Semiconfluent (60–80%) cells grown on 35-mm culture dishes (Corning catalog no. 430165) were loaded for 40 min at 37°C with 5 μM Fura-2 AM in the same Krebs solution used for whole lens experiments. After five washes, the dish with Fura-2 AM-loaded cells was mounted on an open perfusion mini-incubator (Harvard Apparatus, model PDMI-2) attached to the stage of an inverted microscope (Nikon Eclipse TS100). The cells were superfused continuously at a rate of 1.5 ml/min. Baseline values for cytoplasmic calcium were obtained by superfusing the cells for 3 min with the control Krebs solution. Cells were then exposed to drugs or hyposmotic solution by switching the feeding reservoir to one with a fixed concentration of the intended drug or with the hyposmotic solution. In some cases cells were exposed to antagonist drugs for 15–20 min at the end of loading period.
The ratio of fluorescence intensity was determined at 340 nm vs. 380 nm using an established method. The experimental fluorescence ratios were converted into free calcium concentration by the system-integrated software by plotting the respective data on the calibration curve. The calibration curve was obtained frequently using a Fura-2 Calcium Calibration Kit supplied by Invitrogen (catalog no. C-3008MP) following the manufacturer's recommended protocol. In brief, fluorescence ratios (340 nm/380 nm) were derived using a set of standards containing 0, 38, 100, 225, 351, 602, and 1,350 nM concentrations of free calcium. A calibration curve was constructed by plotting the Fura-2 fluorescence ratios (F340 nm/F380 nm) against the free calcium concentrations. In each experiment, data from 15–40 individual cells on the dish surface were averaged and considered as n = 1.
Statistical analysis.
Results are expressed as means ± SE of data from a specified number of independent experiments. Statistical comparison was made by one-way analysis of variance followed by the Bonferroni post hoc multiple-comparison test. A probability (P) value of <0.05 was considered significant.
RESULTS
TRPV4 expression.
Recently, we published data showing that intact porcine lenses release ATP when exposed to hyposmotic solution. In several tissues it has been reported that TRPV4 channels play a role in the response to a reduction in extracellular osmolarity. TRPV4 protein expression in the porcine lens epithelium was examined by Western blot analysis of lens epithelial cell homogenate, and a distinct immunoreactive band was observed (Fig. 1). When the TRPV4 antibody was incubated at room temperature (1 h) with a TRPV4 blocking peptide (peptide derived from residues 850 to the COOH terminus of mouse TRPV4), the antibody failed to recognize the band (Fig. 1).
Fig. 1.
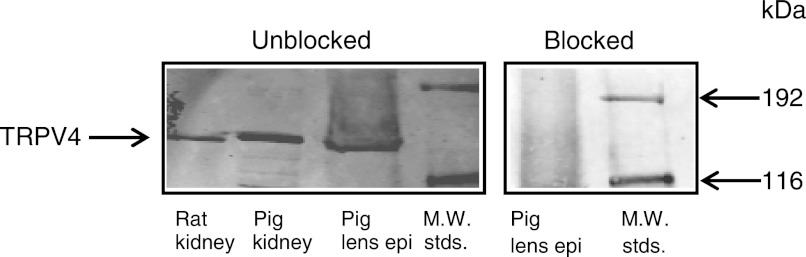
Western blot detection of the transient receptor potential vanilloid 4 (TRPV4) channel in porcine lens epithelium (epi). Rat kidney and pig kidney were used as positive controls. Either 200 μg of lens epithelium or 100 μg of kidney homogenate protein was loaded to each lane. When the primary antibody was blocked with the specific antigen peptide (right), it failed to recognize the TRPV4 band in the lens epithelium.
ATP release.
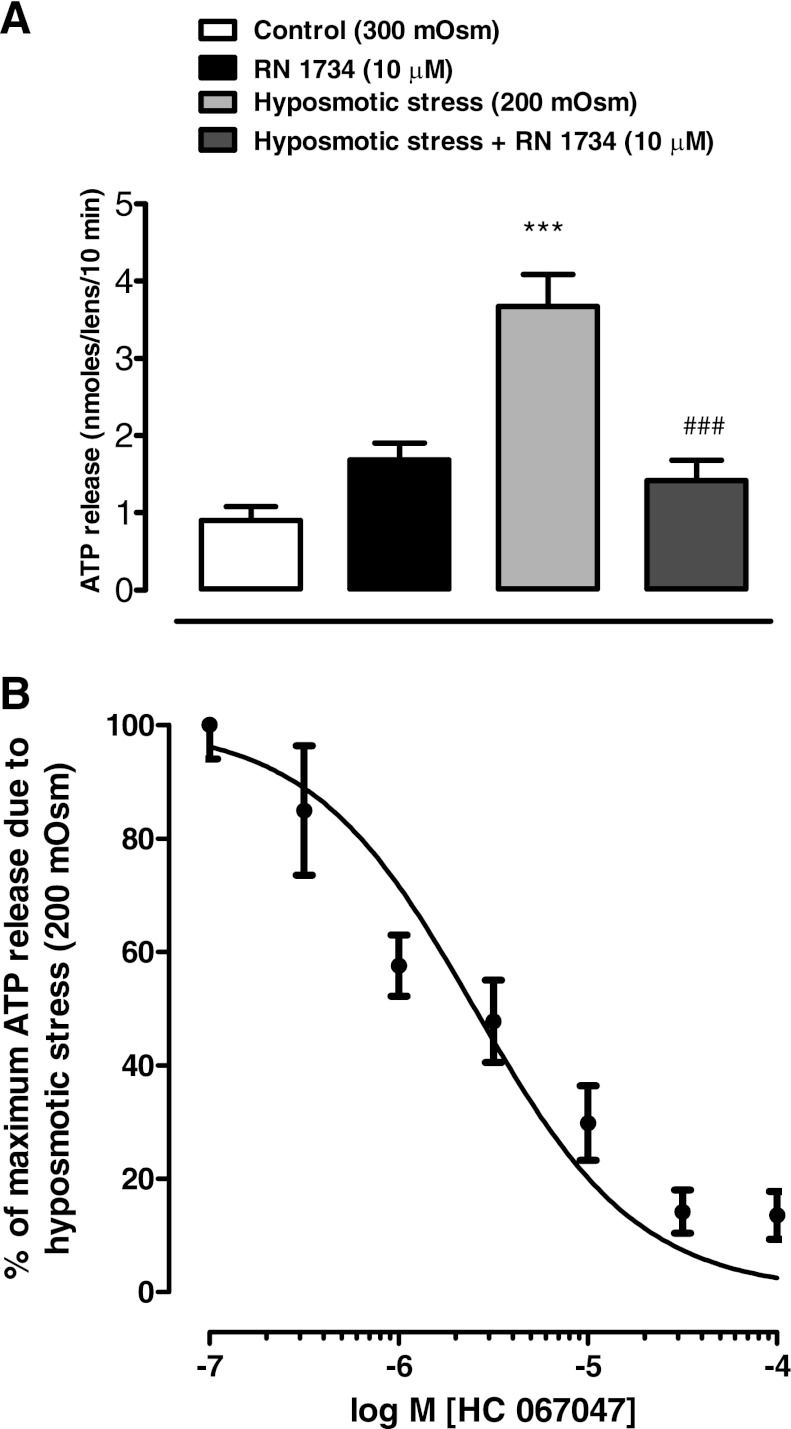
To examine the involvement of TRPV4 in the release of ATP, intact lenses were incubated in hyposmotic solution (200 mosM) for 10 min in the absence or presence of a TRPV4 antagonist, RN 1734, added 20 min before exposure of the lenses to hyposmotic solution, and in the continued presence of RN 1734. The concentration of ATP in the bathing medium increased more than threefold when lenses were exposed to hyposmotic solution, and 10 μM RN 1734 eliminated the increase (Fig. 2A). A different TRPV4 inhibitor, HC 067047, was effective in the submicromolar concentration range. The increased release of ATP from lenses challenged with hyposmotic solution was inhibited by HC 067047 in a concentration-dependent manner with an IC50 of 2.5 μM (Fig. 2B).
Fig. 2.
The influence of TRPV4 antagonists RN 1734 and HC 067047 on the ATP release response to hyposmotic Krebs solution (200 mosM). Intact lenses were exposed to hyposmotic Krebs solution for 10 min in the presence or absence of the TRPV4 antagonist added 20 min beforehand: RN 1734 (10 μM) (A); HC 067047 (0.1–100 μM) (B). Using the data in B, an IC50 of 2.5 μM was calculated for inhibition of hyposmotic solution-induced ATP release by HC 067047. ATP in the bathing solution was measured by luciferin-luciferase assay. Values are means ± SE of results from 6–8 lenses. ***P < 0.001, significant difference from control; ###P < 0.001, significant difference from hyposmotic treatment.
Hemichannel opening.
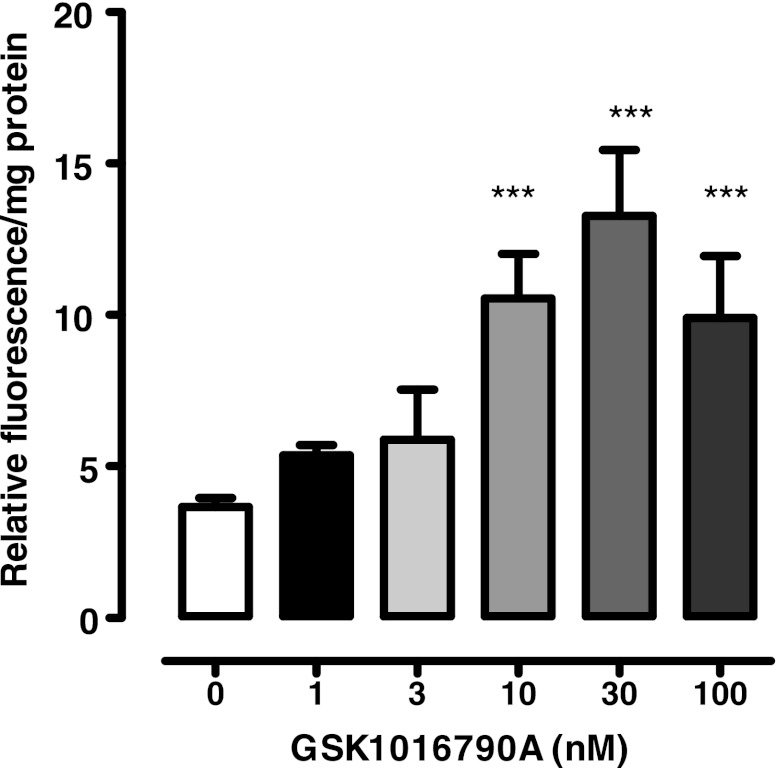
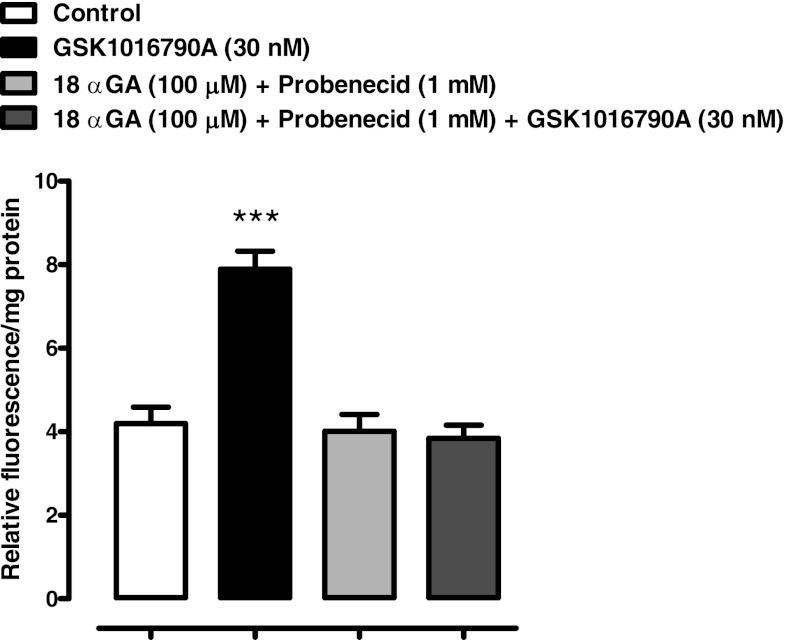
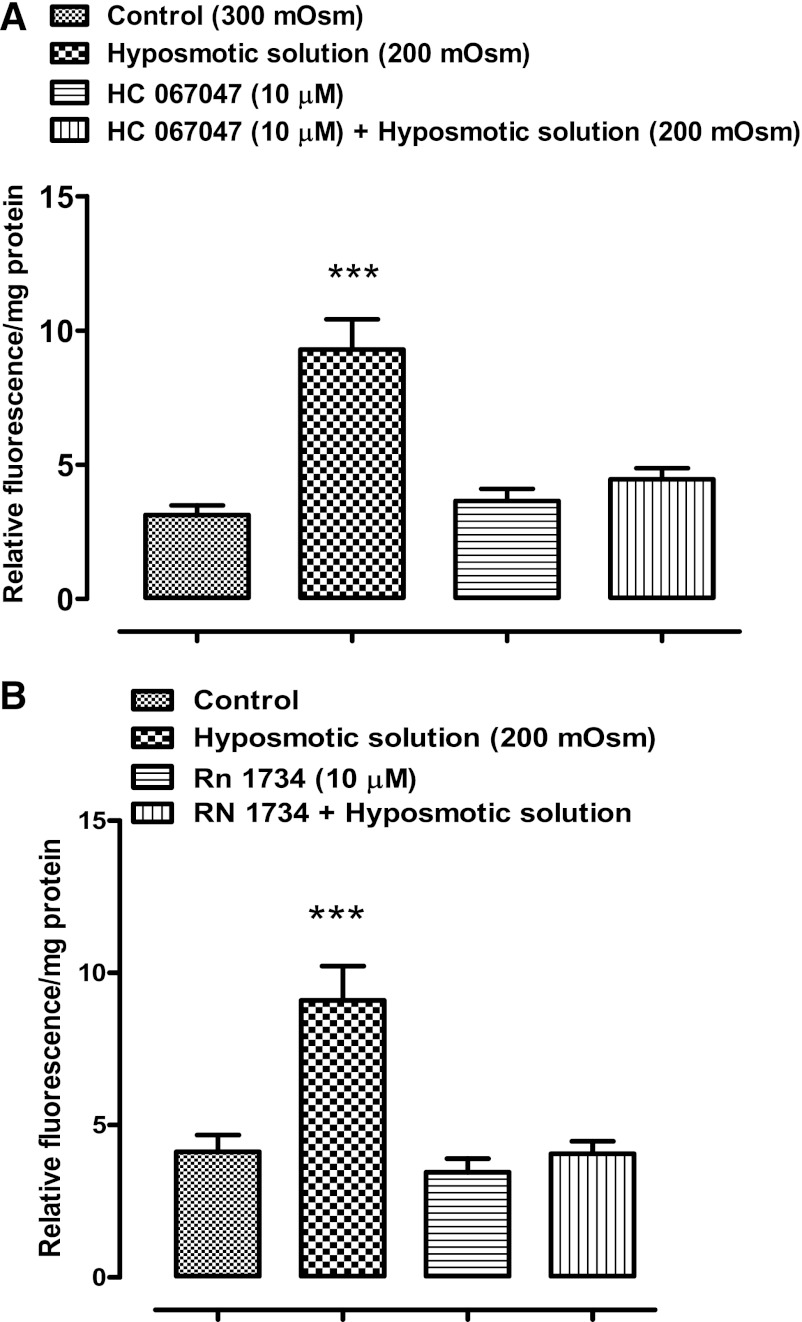
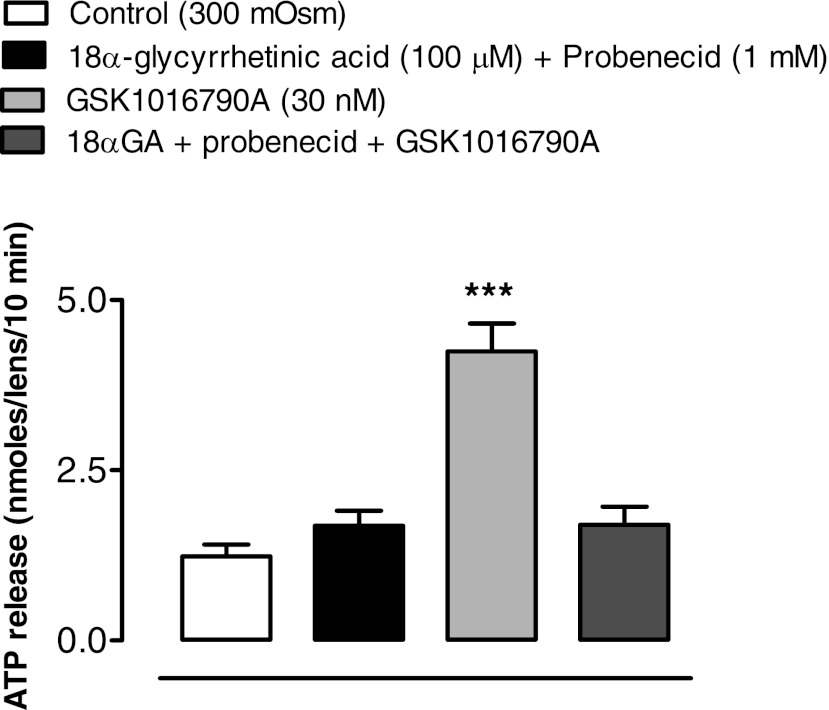
The ability of RN 1734 or HC 067047 to inhibit ATP release from osmotically stressed lenses could be explained if the TRPV4 channel forms a conduit for the exit of cellular ATP. However, uptake studies with PI (MW 668.4) point to a mechanism that involves TRPV4-mediated opening of a pathway that allows the passage of large molecules. Intact lenses were incubated for 20 min in the presence of 25 μM PI. When lenses in isosmotic solution (300 mosM) were exposed to GSK1016790A (1–100 nM), a potent TRPV4 agonist (36), PI entry to the lens epithelium increased significantly at an agonist concentration of 10 nM or higher (Fig. 3). With 30 nM, the increase was more than twofold. Importantly, agonist-induced entry of PI was suppressed completely by a mixture of two hemichannel blockers, 18α-glycyrrhetinic acid (100 μM) plus probenecid (1 mM) (Fig. 4). PI uptake was also increased more than twofold in the epithelium of intact lenses that had been incubated for 20 min in hyposmotic solution. The increase of PI in the epithelium did not occur when lenses were exposed to hyposmotic solution in the presence of a TRPV4 antagonist, HC 067047 (10 μM) (Fig. 5A) or RN 1734 (10 μM) (Fig. 5B). In isosmotic solution, GSK1016790A (30 nM) elicited a greater than twofold increase of ATP release by intact lenses and the response was completely suppressed by the mixture of 18α-glycyrrhetinic acid plus probenecid (Fig. 6).
Fig. 3.
The influence of TRPV4 agonist GSK1016790A on propidium iodide (PI) uptake by the epithelium of lenses exposed to isosmotic Krebs solution (300 mosM). Intact lenses were exposed to 25 μM PI in isosmotic Krebs solution for 20 min in the presence or absence of GSK1016790A (1–100 nM). Values are means ± SE of results from 8 lenses. ***P < 0.001, significant difference from control.
Fig. 4.
The influence of hemichannel blockers 18α-glycyrrhetinic acid (18αGA) and probenecid on the PI uptake response to TRPV4 agonist GSK1016790A by lenses exposed to isosmotic (300 mosM) Krebs solution. Intact lenses were exposed to isosmotic solution containing 30 nM GSK1016790A for 20 min in the presence or absence of a mixture of hemichannel inhibitors 18α-glycyrrhetinic acid (100 μM) and probenecid (1 mM), added 20 min beforehand. Then the epithelium was removed, homogenized, and assayed for PI. Values are means ± SE of results from 6–8 lenses. ***P < 0.001, significant difference from control.
Fig. 5.
The influence of TRPV4 inhibitors HC 067047 and RN 1734 on the PI uptake response to hyposmotic Krebs solution (200 mosM). Intact lenses were exposed to hyposmotic solution for 10 min in the presence or absence of either 10 μM HC 067047 (A) or 10 μM RN 1734 (B), added 20 min beforehand. Then the epithelium was removed, homogenized, and assayed for PI. Values are means ± SE of results from 6–9 lenses. ***P < 0.001, significant difference from control.
Fig. 6.
The influence of hemichannel blockers 18α-glycyrrhetinic acid and probenecid on the ATP release response to TRPV4 agonist GSK1016790A in isosmotic Krebs solution (300 mosM). Intact lenses were exposed to isosmotic Krebs solution containing 30 nM GSK1016790A in the presence or absence of a mixture of hemichannel blockers 18α-glycyrrhetinic acid (100 μM) and probenecid (1 mM) added 20 min beforehand. ATP in the bathing solution was then measured by luciferin-luciferase assay. Values are means ± SE of results from 6–8 lenses. ***P < 0.001, significant difference from control.
Na-K-ATPase activity.
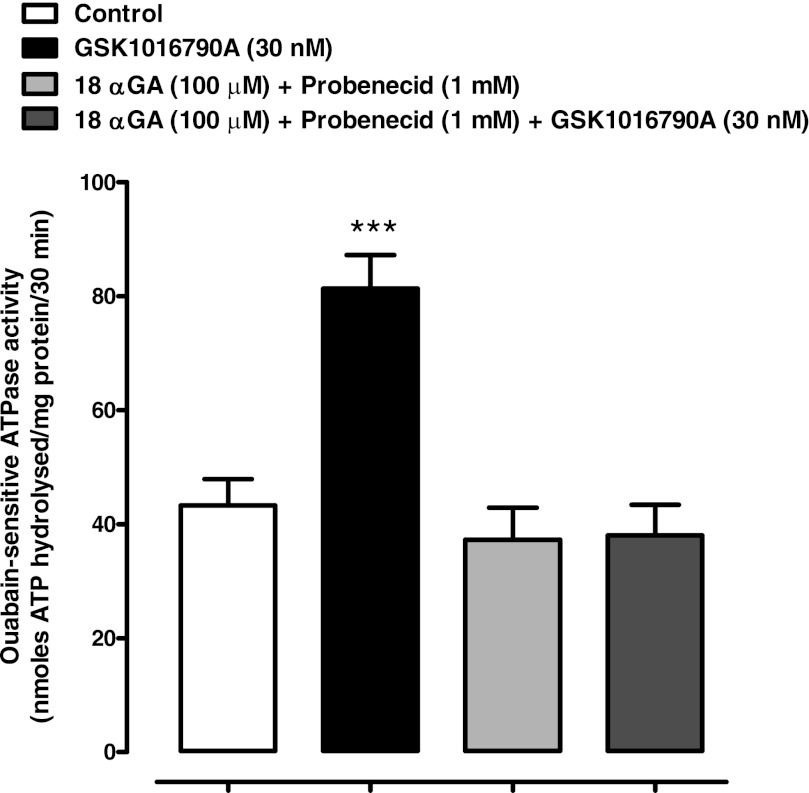
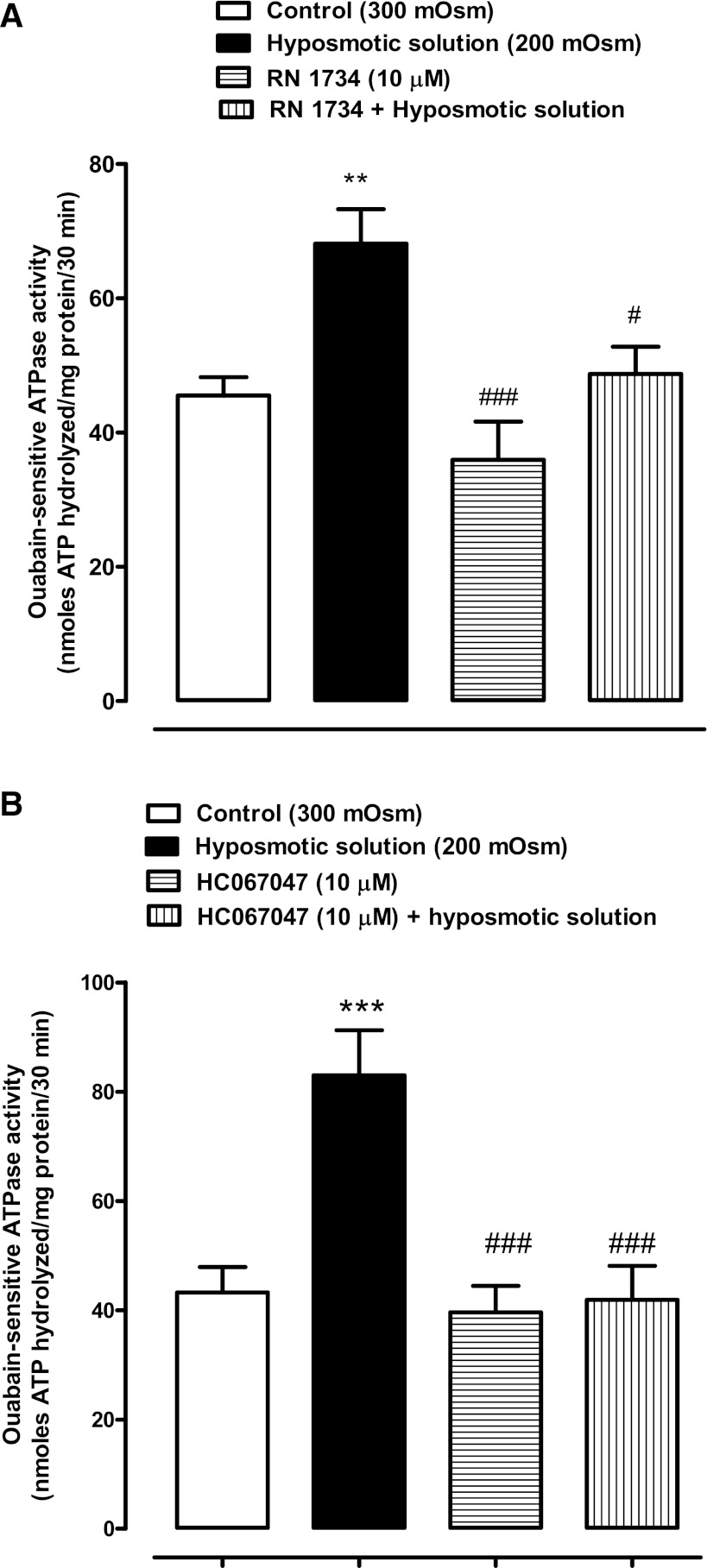
ATP is a potent extracellular signaling molecule, and ATP released in response to hyposmotic stress causes detectable changes in the lens. By activating purinergic receptors, it was shown previously to elicit an increase in Na-K-ATPase activity (32). Exposure of the lens to the TRPV4 agonist GSK1016970A elicits ATP release and PI uptake responses that are qualitatively comparable to the responses observed when lenses are exposed to hyposmotic solution. Thus, to explore the involvement of TRPV4 in the activity of Na-K-ATPase, studies were carried out to examine Na-K-ATPase activity in lenses treated with GSK1016970A. Na-K-ATPase activity was measured in the epithelium of lenses that had been exposed to 30 nM GSK1016970A for 10 min under control (300 mosM) osmotic conditions. A significant increase (∼100%) of Na-K-ATPase activity was observed in the epithelium of GSK1016970A-treated lenses. We reasoned that if the Na-K-ATPase response to TRPV4 activation involves hemichannel opening, the response should be prevented by hemichannel blockade. This was the case. The Na-K-ATPase increase did not occur in lenses exposed to GSK1016970A in the presence of a mixture of 18α-glycyrrhetinic acid and probenecid (Fig. 7). To further examine the role of TRPV4, we studied the ability of two different TRPV4 antagonists to prevent the hypotonic solution-induced increase in Na-K-ATPase activity. At a concentration of 10 μM, RN 1734 prevented the increase of Na-K-ATPase activity in the epithelium of lenses subjected to hyposmotic solution (Fig. 8A). Similarly, a different TRPV4 inhibitor, HC 067047, also abolished the increase of Na-K-ATPase activity elicited by hyposmotic solution (Fig. 8B). Added alone, neither RN 1734 nor HC 067047 significantly altered Na-K-ATPase activity.
Fig. 7.
The influence of hemichannel inhibitors 18α-glycyrrhetinic acid and probenecid on the Na-K-ATPase response to the TRPV4 agonist GSK1016790A in isosmotic solution (300 mosM). Intact lenses were exposed to GSK1016790A (30 nM) for 10 min in the presence or absence of a mixture of hemichannel inhibitors 18α-glycyrrhetinic acid (100 μM) and probenecid (1 mM) added 20 min beforehand. Then the epithelium was removed, homogenized, and assayed for Na-K-ATPase activity. Values are means ± SE of results from 6–8 lenses. ***P < 0.001, significant difference from control.
Fig. 8.
The influence of TRPV4 inhibitors RN 1734 and HC 067047 on the Na-K-ATPase response to hyposmotic Krebs solution (200 mosM). Intact lenses were exposed to hyposmotic solution for 10 min in the presence or absence of 10 μM RN 1734 (A) or 10 μM HC 067047 (B), added 20 min beforehand. Then the epithelium was removed, homogenized, and assayed for Na-K-ATPase activity. Values are means ± SE of results from 4–6 lenses. **,***P < 0.001, significant difference from control; #,###P < 0.001, significant difference from hyposmotic treatment.
Src family tyrosine kinase activation.
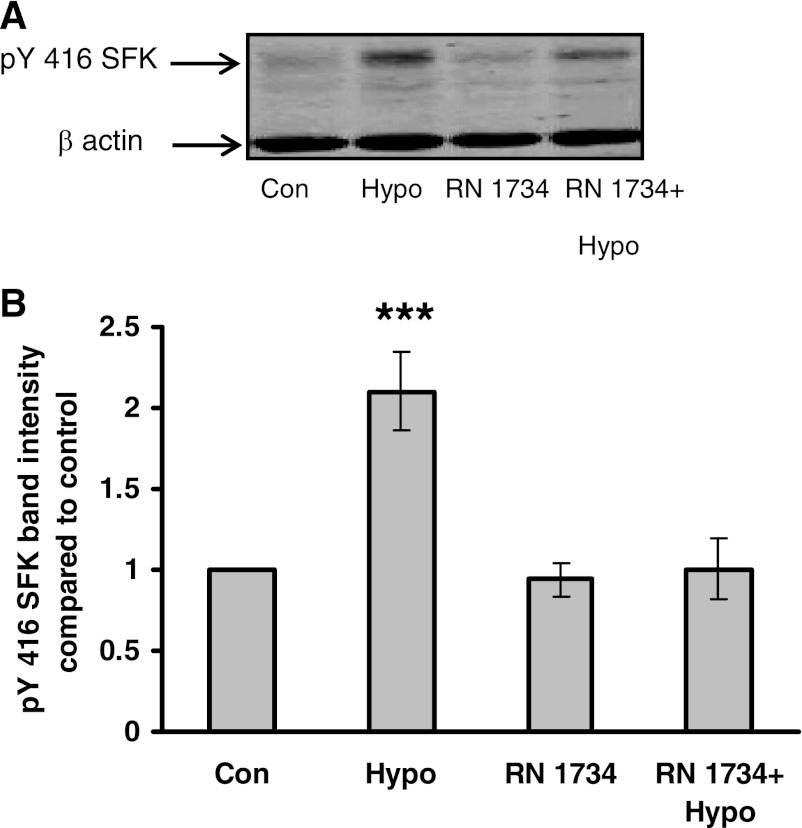
Previously, exposure of the lens to hyposmotic solution was found to cause Src family tyrosine kinase (SFK) activation as evidenced by the increased band density of phospho-Y416-SFK (32). Studies were conducted to examine the effects of RN 1734 on SFK activation as evidenced by Western blot analysis using an antibody to detect the phospho-Y416-SFK in the epithelium of lenses that had been exposed to hyposmotic solution for 2 min. Earlier we found that exposure to hyposmotic solution for 2 min produced peak increase (∼100%) in phospho-Y416-SFK band density (32). The increase did not occur when lenses were exposed to hyposmotic solution in the presence of the TRPV4 inhibitor RN 1734 (10 μM) (Fig. 9).
Fig. 9.
The influence of TRPV4 inhibitor RN 1734 on the Src family kinase (SFK) phosphorylation response to hyposmotic Krebs solution (200 mosM). Intact lenses were exposed to hyposmotic solution for 2 min in the presence or absence of RN 1734 (10 μM) added 20 min beforehand, after which the epithelium was removed, homogenized, and subjected to Western blot analysis for SFK phosphorylation at Y416. β-Actin was probed as a loading control. A: representative Western blot. Con, control; Hypo, hyposmotic solution. B: relative phospho-SFK (pY416 SFK) band density (compared with control). Values are means ± SE of results from 3 independent experiments. ***P < 0.001, significant difference from control.
Calcium entry.
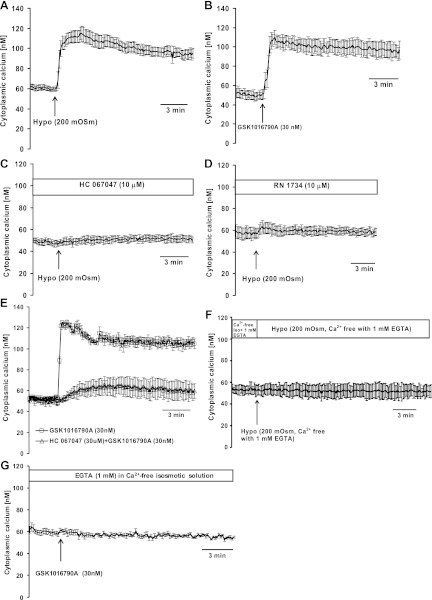
To clarify the role of TRPV4-mediated calcium entry in the responses to hyposmotic solution, studies were conducted in intact lenses as well as in cultured lens epithelium where cytoplasmic calcium concentration can be measured. An immediate increase (∼100%) of cytoplasmic calcium concentration was observed in cultured lens epithelial cells when bathing solution osmolarity was reduced from 300 to 200 mosM (Fig. 10A) or when cells were exposed to the TRPV4 agonist GSK1016790A in isosmotic bathing solution (300 mosM) (Fig. 10B). In both cases, cytoplasmic calcium remained elevated for the duration of the experiment (∼20 min). In the presence of TRPV4 inhibitors HC 067047 (10 μM, Fig. 10C) or RN 1734 (10 μM, Fig. 10D), the cytoplasmic calcium response to hyposmotic solution or to GSK1016790A in isosmotic solution (Fig. 10E) was almost fully suppressed. When the experiments were conducted in calcium-free solutions that contained 1 mM EGTA, the cytoplasmic calcium response to hyposmotic solution (Fig. 10F) or to GSK1016790A in isosmotic solution (Fig. 10G) also was almost completely eliminated.
Fig. 10.
Cytoplasmic calcium concentration measured in cultured porcine lens epithelium loaded with Fura-2 AM. A: cytoplasmic calcium increase in response to hyposmotic solution (200 mosM). B: calcium response to TRPV4 agonist GSK1016790A in isosmotic solution (300 mosM). C and D: calcium response to hyposmotic solution in the presence of TRPV4 inhibitor HC 067047 (10 μM) or RN 1734 (10 μM), added 20 min beforehand. E: calcium response to TRPV4 agonist GSK1016790A (30 nM) in isosmotic solution in the presence of TRPV4 antagonist HC 067047. F and G: effect of calcium-free Krebs solution containing EGTA (1 mM) on the calcium response to hyposmotic solution (F) and GSK1016790A (30 nM) in isosmotic solution (G). Data from 15–30 individual cells were averaged and considered as n = 1. Results are means ± SE of 4–6 independent experiments.
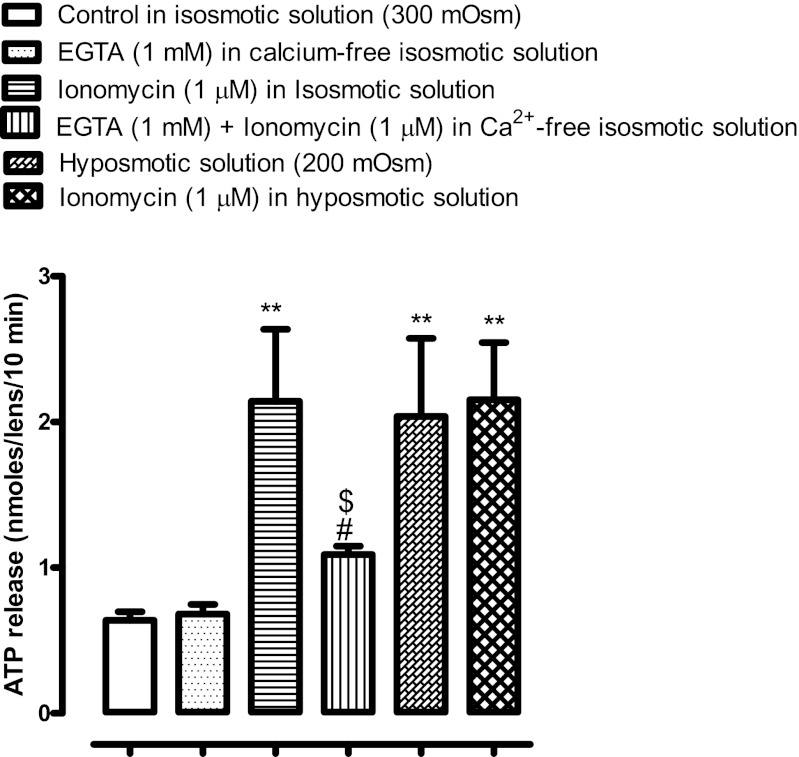
When intact lenses were exposed to isosmotic solution for 10 min in the presence of ionomycin (1 μm), which is known to increase cytoplasmic calcium, ATP release was increased by more than twofold. Consistent with the notion that calcium entry is a requirement for ATP release, the magnitude of ATP release from ionomycin-treated lenses was significantly reduced when calcium was removed from the bathing medium (calcium-free/EGTA solution). Removal of calcium from the bathing medium in itself did not cause any significant increase in ATP release in isosmotic solution. The magnitude of ATP release by lenses exposed to ionomycin in isosmotic solution was similar to that induced by hyposmotic solution (∼twofold in both cases). Crucially, the magnitude of ATP release was no greater when lenses were exposed simultaneously to ionomycin and hyposmotic solution (Fig. 11). When lenses were exposed simultaneously to hyposmotic conditions and calcium-free/EGTA solution, the ATP release results were inconsistent (data not shown).
Fig. 11.
The influence of ionomycin on ATP release. Intact lenses were exposed to ionomycin (1 μM) for 10 min in isosmotic (300 mosM) Krebs solution with or without calcium (calcium-free/1 mM EGTA), then ATP was measured in the bathing solution. One group of lenses was exposed to hyposmotic solution (200 mosM) alone. A different group of lenses was exposed to isosmotic calcium-free/1 mM EGTA solution. Another group of lenses was exposed to ionomycin in hyposmotic solution. Control lenses were incubated in isosmotic Krebs solution and did not receive ionomycin. Values are means ± SE of results from 6–8 lenses. **P < 0.001, significant difference from control; #P < 0.001, significant difference from ionomycin treatment; $P < 0.001, significant difference from hyposmotic treatment.
DISCUSSION
TRPV4 expression was observed in lens epithelium and functional studies with intact porcine lenses point to a critical role for TRPV4 channels in the response of the lens to hyposmotic shock. The TRPV4 inhibitor RN 1734 prevented ATP release from intact lenses subjected to hyposmotic shock. ATP released from lenses exposed to hyposmotic solution was previously shown to stimulate purinergic receptors in the lens epithelium, initiating an Src family tyrosine kinase-dependent stimulation of Na-K-ATPase activity (32). Consistent with blockade of ATP release, TRPV4 inhibition prevented the change of Na-K-ATPase activity in lenses exposed to hyposmotic conditions and TRPV4 activation in isosmotic solution with an agonist (GSK1016790A) released ATP and stimulated Na-K-ATPase activity.
There are few reports on the TRPV4-dependence of ATP release in cells subjected to hyposmotic stress (16, 33), but it is known that in several cell types, heat and mechanical stimuli elicit ATP release by a TRPV4-dependent mechanism (24, 25). Even though ATP release is TRPV4-dependent, the TRPV4 channel itself is not necessarily the conduit for ATP exit. Mihara and coworkers (24) proposed heat-activated ATP release from esophageal keratinocytes occurs via exocytosis. However, our recent study on intact porcine lens showed little or no contribution of exocytosis in ATP release in response to hyposmotic stress (32). Mochizuki and coworkers (25) speculated that stretch-activated ATP release from urothelial cells might involve hemichannels. ATP release through connexin and pannexin hemichannels appears to be a widespread phenomenon in many cell types (5, 30, 37) including ocular epithelia, such as corneal endothelium (14) or ciliary epithelium (19). In a recent study this laboratory presented evidence that connexin and pannexin hemichannels contribute to the hyposmotic stress-induced release of ATP from the porcine lens (32). On the basis of current findings, we propose that TRPV4 activation is required for connexin/pannexin-mediated ATP release. Opening of hemichannels upon exposure of the intact lens to hyposmotic solution is consistent with both ATP release and the increased ability of propidium iodide (PI) to enter the lens epithelium. The TRPV4 inhibitors RN 1734 and HC 067047 prevented both ATP release and PI uptake. Furthermore, the TRPV4 agonist GSK1016790A released ATP and elicited PI uptake increase under isosmotic conditions (300 mosM). In addition, hemichannel inhibitors 18α-glycyrrhetinic acid and probenecid suppressed the increase of PI accumulation observed in GSK1016790A-treated lenses. We recognize the possibility that a concentration of 100 μM 18α-glycyrrhetinic acid may be sufficient to inhibit both connexins and pannexins. We also acknowledge the possibility that PI entry may take place in certain regions of the epithelium and, once inside the cell, PI could be transferred to adjacent areas of the epithelium monolayer via gap junctions that are sensitive to 18α-glycyrrhetinic acid. It is noteworthy that a pannexin blocker, probenecid, which is not known to affect connexins, produced ∼70% reduction in PI uptake in comparison to ∼50% reduction caused by 18α-glycyrrhetinic acid (32). Taken together, the findings suggest that activation of TRPV4 channels by hyposmotic stress leads to the opening of hemichannels in lens epithelium. Recent reports in other tissues strongly support our finding in that TRPV4 activation triggers ATP release through connexin 43 and pannexin 1 hemichannels in esophageal epithelial cells (37). Furthermore, in airway epithelium it has been shown that hypotonicity-induced ATP release through pannexin hemichannels is mediated through the transduction of membrane stretch signal by TRPV4 (30). In this context it is worth mentioning that porcine lens epithelium expresses connexin 43, connexin 50, and pannexin 1 (32) and TRPV4.
A rise of cytoplasmic calcium concentration was observed when cultured porcine lens epithelium was exposed to hyposmotic solution. Although they are nonselective, TRPV4 cation channels are reported to be permeable to calcium ions (11). Consistent with requirement for TRPV4 activation in the response to hyposmotic solution, the selective antagonists HC 067047 or RN 1734 almost completely eliminated the calcium rise. Importantly, under isosmotic conditions the TRPV4 agonist GSK1016790A increased cytoplasmic calcium concentration almost to the same extent and this response was also prevented by the TRPV4 antagonists. While questions remain regarding the mechanism responsible for the calcium rise, the results from experiments with cultured lens cells point to the requirement of TRPV4 for entry of calcium from the bathing medium. Calcium free/EGTA Krebs solution prevented the cytoplasmic calcium increase elicited by both hyposmotic solution and by GSK1016790A in isosmotic solution. The cytoplasmic calcium rise may coordinate the cell response to hyposmotic stress, and experiments with intact lenses were consistent with the requirement for calcium entry since eliminating calcium from the bathing solution significantly suppressed ionomycin-induced ATP release in isosmotic solution. Crucially, hyposmotic-solution-induced ATP release could not be further increased by addition of ionomycin. There are still some unanswered questions and more studies are needed to separately identify the contribution of TRPV4 and hemichannels to calcium entry in hyposmotic solution. It should be noted that when we tried to suppress hyposmotic solution-induced ATP release by removing calcium from the bathing medium (calcium-free/EGTA medium), the results were variable and inconsistent. It is possible that lenses are subjected to excessive stress when they are exposed simultaneously to hyposmotic and calcium-free/EDTA conditions. It is also possible that the findings reflect the complex nature of hemichannel responses to changes of intracellular and extracellular calcium concentration. In ventricular myocytes, as well as in HEK293 cells that overexpress connexin 43, it has been shown that calcium-free extracellular medium triggers hemichannel opening (17). On the other hand, increased cytoplasmic calcium has been reported to promote opening of connexin 32, connexin 43, and pannexin 1 hemichannels (6, 7, 21, 38). However, there are experiments that show pannexin 1 is independent of cytoplasmic calcium concentration (20, 22). Furthermore, the involvement of cytoplasmic calcium in opening connexin 43 hemichannels appears complex, with opening observed in response to increased calcium up to 500 nM but disappearance of the response to larger cytoplasmic calcium transients (7). Porcine lens epithelium expresses connexin 43, connexin 50, and pannexin 1 (32) and further experiments are needed to examine the phenomenon of hemichannel opening following the cytoplasmic calcium increase caused by hyposmotic stress-induced activation of TRPV4 channels.
The epithelium monolayer accounts for very little of the bulk of the lens yet Na-K-ATPase-mediated active cation transport by the lens epithelium is crucial for ionic homeostasis of the entire lens cell mass (9). Na-K-ATPase activity in the epithelium is subject to regulation by Src family tyrosine kinases (SFKs). By causing release of ATP that subsequently activates P2Y purinergic receptors, leading to SFK activation, hyposmotic stress has been found to increase Na-K-ATPase activity in the lens epithelium (32). In keeping with the notion that TRPV4 channel activation is necessary for hemichannel-mediated ATP release to occur, the TRPV4 inhibitors RN 1734 or HC 067047 both were found to prevent SFK activation as well as the increase in Na-K-ATPase in lenses exposed to hyposmotic solution. Moreover, the TRPV4 agonist GSK1016790A elicited an increase of Na-K-ATPase activity that was abolished by hemichannel blockers 18α-glycyrrhetinic acid and probenecid. The results point to a pivotal role for TRPV4 activation as an initial event in a multistep response of the lens to hyposmotic stress.
Exposure of the lens to hyposmotic solution causes water entry. Similarly, water accumulation by the lens occurs as a consequence of osmolyte (sorbitol) accumulation in human diabetic cataract as well as animal models of diabetic cataract. Thus it is important to understand the nature of osmotic responses in the lens even though it is inconceivable that lenses are exposed in vivo to osmolarity as low as 200 mosM. Because hyposmotic solution causes swelling of the entire lens structure (18) as well as individual lens cells (3), we have no basis on which to distinguish a strictly osmotic response from a response to mechanical stress. Recent studies have pointed to the importance of TRPV4 association with the cytoskeleton, and it has been suggested that mechanosensitivity could arise in part because microtubules are a regulator of TRPV4 channel activity (15). Since osmotic disturbances cause mechanical stress and since TRPV4 channels are shown to be both osmo- and mechanosensitive, we speculate that TRPV4 responses could be involved in diabetic cataract.
GRANTS
This research was supported by a grant from the National Institutes of Health (Grant NIH EY009532).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.S. and N.A.D. conception and design of the research; M.S. and A.M. performed the experiments; M.S. and A.M. analyzed the data; M.S., A.M., and N.A.D. interpreted the results of the experiments; M.S. and A.M. prepared the figures; M.S. and N.A.D. drafted the manuscript; M.S. and N.A.D. edited and revised the manuscript; M.S. and N.A.D. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank the University of Arizona Meat Science Laboratory, West Valley Meat Processing, Buckeye, Phoenix and Hatfield Quality Meat, Pennsylvania for continued willingness to supply porcine eyes.
REFERENCES
- 1.Andersson RM, Aizman O, Aperia A, Brismar H. Modulation of Na+,K+-ATPase activity is of importance for RVD. Acta Physiol Scand 180: 329–334, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Becker D, Blase C, Bereiter-Hahn J, Jendrach M. TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci 118: 2435–2440, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Chimote AA, Adragna NC, Lauf PK. Ion transport in a human lens epithelial cell line exposed to hyposmotic and apoptotic stress. J Cell Physiol 223: 110–122, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Clarke TC, Williams OJS, Martin PEM, Evans WH. ATP release by cardiac myocytes in a simulated ischaemia model: inhibition by a connexin mimetic and enhancement by an antiarrhythmic peptide. Eur J Pharmacol 605: 9–14, 2009 [DOI] [PubMed] [Google Scholar]
- 6.De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J 25: 34–44, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Vuyst E, Wang N, Decrock E, De Bock M, Vinken M, Van Moorhem M, Lai C, Culot M, Rogiers V, Cecchelli R, Naus CC, Evans WH, Leybaert L. Ca2+ regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium 46: 176–187, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Delamere NA, Tamiya S. Expression, regulation and function of Na,K-ATPase in the lens. Progress Retinal Eye Res 23: 593–615, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Delamere NA, Tamiya S. Lens ion transport: from basic concepts to regulation of Na,K-ATPase activity. Exp Eye Res 88: 140–143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Progress Biophys Mol Biol 103: 2–17, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA 107: 19084–19089, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan HC, Zhang X, McNaughton PA. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem 284: 27884–27891, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes J, Lorenzo IM, Andrade YN, Garcia-Elias A, Serra SA, Fernandez-Fernandez JM, Valverde MA. IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. J Gen Physiol 131: i2, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci 46: 1208–1218, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Goswami C, Kuhn J, Heppenstall PA, Hucho T. Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLos One 5: e11654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, Larusso NF. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci USA 104: 19138–19143, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem 274: 236–240, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Kong CW, Gerometta R, Alvarez LJ, Candia OA. Changes in rabbit and cow lens shape and volume upon imposition of anisotonic conditions. Exp Eye Res 89: 469–478, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li A, Leung CT, Peterson-Yantorno K, Mitchell CH, Civan MM. Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am J Physiol Cell Physiol 299: C1308–C1317, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Mitchell CH, Civan MM. Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow. J Cell Physiol 227: 172–182, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 580: 239–244, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther 328: 409–418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandal A, Shahidullah M, Delamere NA. Hydrostatic pressure-induced release of stored calcium in cultured rat optic nerve head astrocytes. Invest Ophthalmol Vis Sci 51: 3129–3138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol 589: 3471–3482, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284: 21257–21264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol 286: C195–C205, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Pan Z, Yang H, Mergler S, Liu H, Tachado SD, Zhang F, Kao WWY, Koziel H, Pleyer U, Reinach PS. Dependence of regulatory volume decrease on transient receptor potential vanilloid 4 (TRPV4) expression in human corneal epithelial cells. Cell Calcium 44: 374–385, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollreisz A, Schmidt-Erfurth U. Diabetic cataract-pathogenesis, epidemiology, and treatment. J Ophthalmol 2010; 608751, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robison WG, Jr, Houlder N, Kinoshita JH. The role of lens epithelium in sugar cataract formation. Exp Eye Res 50: 641–646, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O'Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem 286: 26277–26286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shahidullah M, Delamere NA. NO donors inhibit Na,K-ATPase activity by a protein kinase G-dependent mechanism in the nonpigmented ciliary epithelium of the porcine eye. Br J Pharmacol 148: 871–880, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahidullah M, Mandal A, Beimgraben C, Delamere NA. Hyposmotic stress causes ATP release and stimulates Na,K-ATPase activity in porcine lens. J Cell Physiol 227: 1428–1437, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Silva GB, Garvin JL. TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. Am J Physiol Renal Physiol 295: F1090–F1095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Analyt Biochem 150: 76–85, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Tamiya S, Dean WL, Paterson CA, Delamere NA. Regional distribution of Na,K-ATPase activity in porcine lens epithelium. Invest Ophthalmol Vis Sci 44: 4395–4399, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ESR, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: part I. J Pharmacol Exp Ther 326: 432–442, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Ueda T, Shikano M, Kamiya T, Joh T, Ugawa S. The TRPV4 channel is a novel regulator of intracellular Ca2+ in human esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol 301: G138–G147, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol 75: 1262–1279, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Wallick ET, Schwartz A. Interaction of cardiac glycosides with Na+,K+-ATPase. Methods Enzymol 156: 201–213, 1988 [DOI] [PubMed] [Google Scholar]
- 40.Xie ZJ, Wang YH, Ganjeizadeh M, McGee R, Jr, Askari A. Determination of total (Na+ + K+)-ATPase activity of isolated or cultured cells. Analyt Biochem 183: 215–219, 1989 [DOI] [PubMed] [Google Scholar]