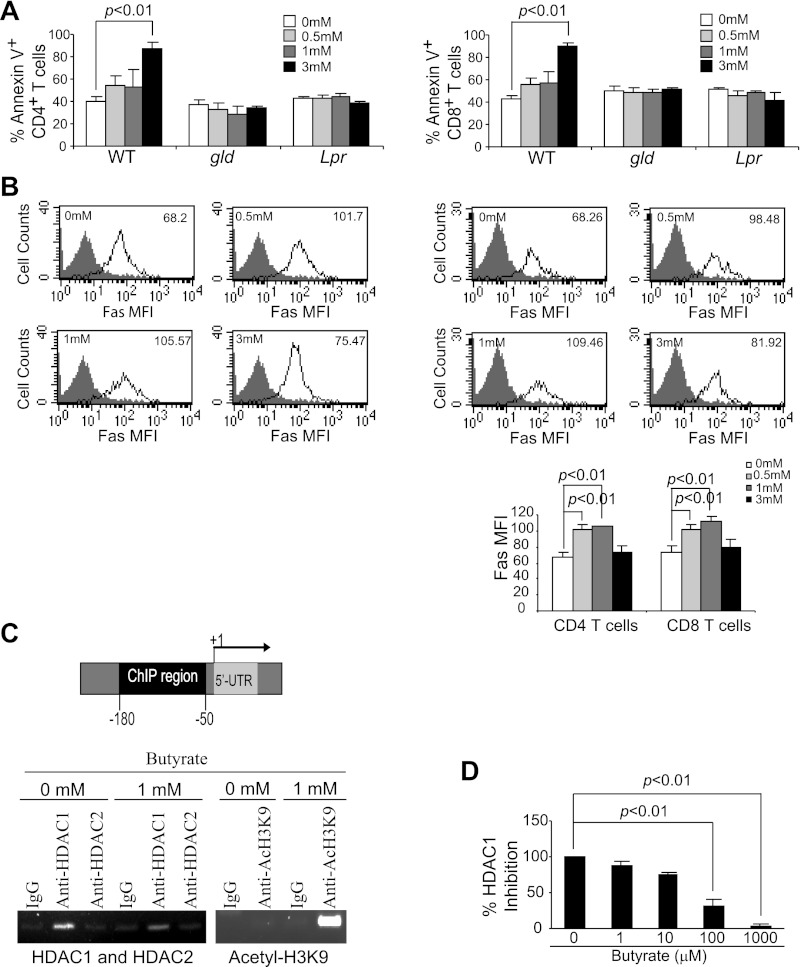
Fig. 4.
Butyrate inhibits Fas promoter-bound histone deacetylase 1 (HDAC1) to induce Fas promoter hyperacetylation and Fas upregulation in T cells. A: induction of T cell apoptosis by butyrate is Fas dependent. Spleen cells from Fasgld and Faslpr were cultured in anti-CD3/CD28 mAb-coated plates with butyrate for 24 h. Cells were then collected and analyzed for apoptosis of CD4+ (A) and CD8+ (B) T cells as in Fig. 2. B: Fas expression level is upregulated by butyrate treatment. Spleen cells were cultured in anti-CD3/CD28-coated plates in the absence or presence of various concentrations of butyrate for 24 h. Cells were then stained with CD4- and Fas-specific mAbs (top, left) or CD8- and Fas-specific mAbs (top, right) and analyzed by flow cytometry. The Fas protein level is represented by the mean fluorescent intensity. The butyrate concentrations are indicated at the upper left corner of each plot, and the Fas MFI is indicated at the upper right corner of each plot. Shown is 1 representative result of 3 independent experiments. Bottom: Fas MFI in CD4+ and CD8+ T cells after butyrate treatment was quantified and presented. Data are means ± SD. C: HDAC1 association with the Fas promoter and butyrate-induced Fas promoter hyperacetylation. Left: mouse Fas promoter structure showing the chromatin immunoprecipitation (ChIP) PCR amplified region. Right: ChIP analysis of HDAC1, HDAC2, and acetyl-H3K9 association with the mouse Fas promoter. The HDAC1-, HDAC2-, and acetyl-H3K9-bound chromatin fragments were immunoprecipitated with the indicated antibodies and analyzed by PCR using Fas promoter sequence-specific primers. D: inhibition of HDAC1 activity by butyrate. Recombinant human HDAC1 was assayed for its activity using a commercially available kit in the absence or presence of butyrate. WT, wild-type.