Figure 6. ADEP leads to the degradation of newly synthesized proteins prior to folding in bacterial cells.
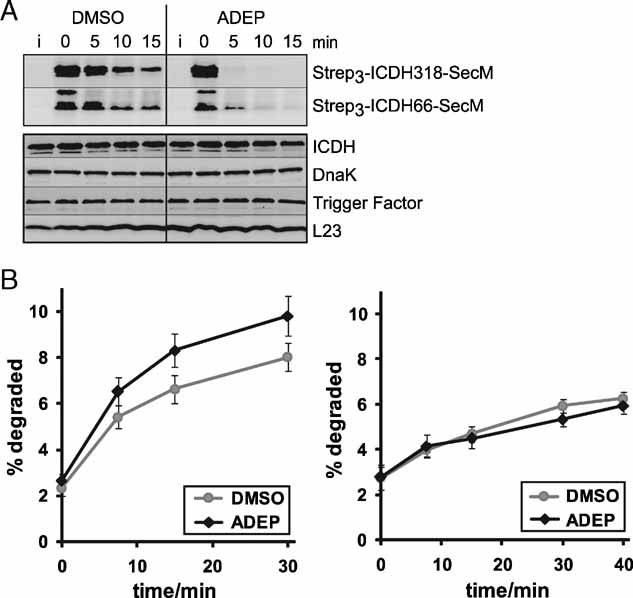
- ADEP causes the degradation of ribosome-arrested nascent polypeptides in vivo. The degradation of two ribosome-arrested nascent chains derived from ICDH (Strep3-ICDH66-SecM, Strep3-ICDH318-SecM) was monitored in ER2566 ΔacrA::kan cells at 37 °C. Two hours after induction (i) of the model constructs, translation was stopped with chloramphenicol and ADEP or DMSO was added (0 min). At the indicated time points, cells were harvested and proteins separated by SDS–PAGE followed by Western blots against the N-terminal Strep-tag (upper panel) and against ICDH, DnaK, Trigger Factor and L23 as controls (lower panel). Only full-length products are depicted.
- The degradation of newly synthesized proteins is increased in the presence of ADEP in vivo. Total protein degradation was determined in HN818 ΔacrA::kan cells in the exponential phase at 37 °C. ADEP1 and DMSO were either added before (left panel) or after (right panel) [35S] methionine pulse labelling. At the denoted time points, aliquots were TCA precipitated and the radioactivity in the TCA-soluble and insoluble fractions was determined by scintillation counting. The amount of degradation is given as percentage of the total cellular radioactivity. Mean values and standard errors of the mean of four to five independent experiments are shown.
