Figure 3. Gene expression and signaling regulation during adult muscle regeneration.
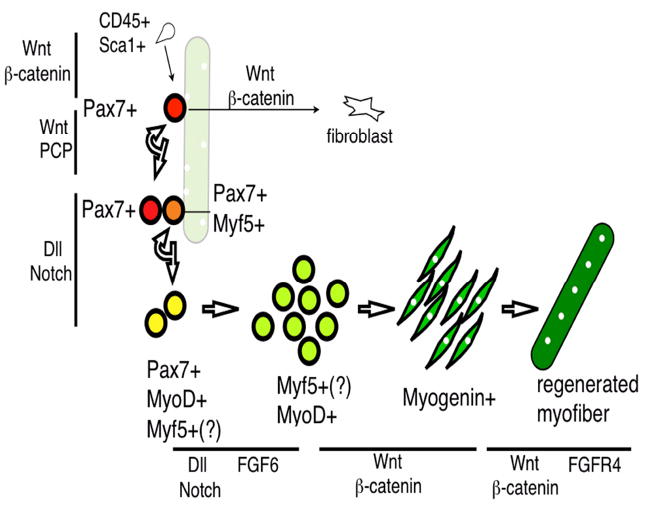
CD34+Sca1+ side population cells can be converted into Pax7+ cells via Wnt/β-catenin signaling. Pax7+ satellite cells (red) are associated with the myofiber (pale green) with peripheral myonuclei (white). Upon injury, Pax7+ cells either go through symmetric division for self-renewal via the Wnt/PCP pathway or asymmetric division to give rise to committed progenitors (Pax7+Myf5+, orange) involving Dll/Notch signaling. These cells expand (as transit amplifying cells) and express MyoD (yellow). Myf5 is presumed (Myf5 (?)) to be expressed in transit amplifying cells and Pax7-MyoD+ myoblasts (yellowish green). Myogenin (green) is turned on in differentiated myocytes, which eventually fuse to form the new myofiber (dark green), which has the characteristic of centrally located myonuclei. Wnt/β-catenin can also convert Pax7+ cells into fibroblasts. There are at least two steps of muscle regeneration involving FGF signaling: FGF6 affects MyoD+ cells, while FGF4R4 affects myofiber number and size. Gene expression for each stage is confined to those covered in the text and does not represent a full list. Wnt, FGF, and Notch pathways that regulate specific steps of myogenic progression are represented by black lines next to the defined steps.
