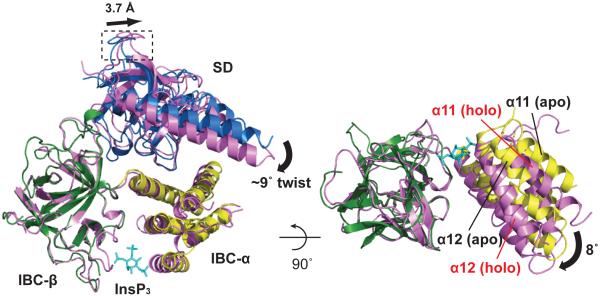
Figure 2. InsP3-evoked conformational changes.
Superposition of apo-NTCysless (SD, blue; IBC-α, yellow; IBC-β, green) and InsP3-bound NTCysless (3.6 Å resolution, magenta) by overlaying IBC-β domain. InsP3 binding causes the SD to rotate towards the IBC accompanied by a swing approximately perpendicular to the IBC ‘clam closure’. This twist (curved arrow) is measured as the angular difference between the SD arm helices in the apo- and bound states (~9°). Movement of the HS-loop (boxed) shows the distance between α-carbons of Y167 (~3.7 Å). A view rotated 90° about the x-axis is shown at right with only IBCs represented. The interdomain (IBC-β and IBC-α) angular difference between the free and bound states is ~8° (black arrow). Further details of InsP3 binding and its effects on the IBC and α- and β-interfaces in Supplementary Figure 5.