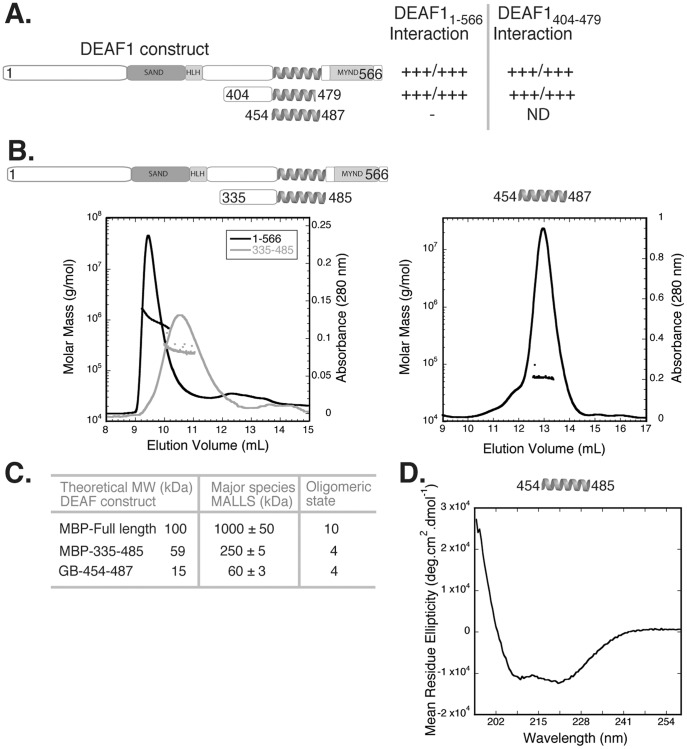
Figure 2. Characterising DEAF1 and the coiled coil domain.
A. Schematic showing DEAF1 constructs used in yeast two-hybrid self-association experiments. Selection was medium/high stringency as in Figure 1D; +++ indicates strong growth, - indicates no growth, ND indicates not determined. B. SEC-MALLS analysis of full length and DEAF1335–485 constructs (left panel) and DEAF1404–479 and the coiled coil domain (right panel). DEAF1 proteins (∼200 µg) were applied to a Superose 12 column with an in line MALLS detector to determine weight-averaged molecular weight in solution. The elution (continuous line) and light-scattering (▪) are shown. C. Summary of the theoretical monomeric and experimentally determined molecular weight of DEAF1 proteins in A and B were used to calculate the oligomeric state. D. Far-UV circular dichroism spectropolarimetry (CD) spectrum of the DEAF1 coiled coil domain.